Online first
Bieżący numer
Archiwum
O czasopiśmie
Polityka etyki publikacyjnej
System antyplagiatowy
Instrukcje dla Autorów
Instrukcje dla Recenzentów
Rada Redakcyjna
Komitet Redakcyjny
Recenzenci
Wszyscy recenzenci
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
Kontakt
Bazy indeksacyjne
Klauzula przetwarzania danych osobowych (RODO)
PRACA PRZEGLĄDOWA
Wpływ wybranych składników pokarmowych oraz stylu życia na funkcję bariery jelitowej człowieka
1
Uniwersytet Medyczny w Białymstoku, Polska
Med Og Nauk Zdr. 2021;27(3):265-271
SŁOWA KLUCZOWE
DZIEDZINY
STRESZCZENIE
Wprowadzenie i cel:
Dysfunkcja bariery jelitowej sprzyja wzmożonej migracji antygenów, bakterii oraz toksyn ze światła jelita do krążenia ogólnego. Stan ten obserwuje się w przebiegu różnych chorób, zarówno o charakterze ostrym, jak i przewlekłym. Modyfikacja funkcji bariery jelitowej może potencjalnie wpływać na przebieg tych chorób i rokowanie pacjentów. Celem niniejszej pracy była ocena wpływu wybranych składników pokarmowych oraz elementów związanych ze stylem życia na funkcję bariery jelitowej człowieka.
Metody przeglądu:
Przeglądu piśmiennictwa dokonano, wykorzystując informacje z baz danych PubMed/MEDLINE oraz ScienceDirect, które ukazały się przed styczniem 2021 roku.
Opis stanu wiedzy:
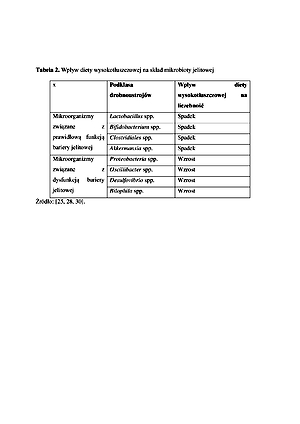
Dieta i styl życia są podstawowymi czynnikami środowiskowymi wpływającymi na funkcję bariery jelitowej. Odpowiednie spożycie błonnika pokarmowego oraz cynku jest niezbędne do zachowania prawidłowych funkcji barierowych. Glutamina jest szczególnie istotnym amino-kwasem w utrzymywaniu bariery nabłonkowej przewodu pokarmowego, a zapotrzebowanie na nią znacząco zwiększa się w przebiegu różnych stanów chorobowych. Dieta wyso-kotłuszczowa oraz alkohol mogą zaburzać strukturę bariery jelitowej, co może nieść negatywne skutki zdrowotne. Gluten wykazuje specyficzny wpływ na przepuszczalność bariery jelitowej poprzez zdolność do uwalniania zonuliny, jednak w warunkach fizjologicznych proces ten jest ściśle kontrolo-wany i prawdopodobnie nie ma istotnego klinicznie znaczenia w przypadku osób zdrowych. Czynniki związane ze stylem życia, tj. stres psychiczny oraz intensywny wysiłek fizyczny, mogą zaburzać funkcję bariery jelitowej, przyczyniając się do zwiększenia przepuszczalności jelitowej.
Podsumowanie:
Dostępne piśmiennictwo sugeruje niewątpliwie istotny wpływ wielu składników pokarmowych oraz stylu życia na funkcję bariery jelitowej.
Dysfunkcja bariery jelitowej sprzyja wzmożonej migracji antygenów, bakterii oraz toksyn ze światła jelita do krążenia ogólnego. Stan ten obserwuje się w przebiegu różnych chorób, zarówno o charakterze ostrym, jak i przewlekłym. Modyfikacja funkcji bariery jelitowej może potencjalnie wpływać na przebieg tych chorób i rokowanie pacjentów. Celem niniejszej pracy była ocena wpływu wybranych składników pokarmowych oraz elementów związanych ze stylem życia na funkcję bariery jelitowej człowieka.
Metody przeglądu:
Przeglądu piśmiennictwa dokonano, wykorzystując informacje z baz danych PubMed/MEDLINE oraz ScienceDirect, które ukazały się przed styczniem 2021 roku.
Opis stanu wiedzy:
Dieta i styl życia są podstawowymi czynnikami środowiskowymi wpływającymi na funkcję bariery jelitowej. Odpowiednie spożycie błonnika pokarmowego oraz cynku jest niezbędne do zachowania prawidłowych funkcji barierowych. Glutamina jest szczególnie istotnym amino-kwasem w utrzymywaniu bariery nabłonkowej przewodu pokarmowego, a zapotrzebowanie na nią znacząco zwiększa się w przebiegu różnych stanów chorobowych. Dieta wyso-kotłuszczowa oraz alkohol mogą zaburzać strukturę bariery jelitowej, co może nieść negatywne skutki zdrowotne. Gluten wykazuje specyficzny wpływ na przepuszczalność bariery jelitowej poprzez zdolność do uwalniania zonuliny, jednak w warunkach fizjologicznych proces ten jest ściśle kontrolo-wany i prawdopodobnie nie ma istotnego klinicznie znaczenia w przypadku osób zdrowych. Czynniki związane ze stylem życia, tj. stres psychiczny oraz intensywny wysiłek fizyczny, mogą zaburzać funkcję bariery jelitowej, przyczyniając się do zwiększenia przepuszczalności jelitowej.
Podsumowanie:
Dostępne piśmiennictwo sugeruje niewątpliwie istotny wpływ wielu składników pokarmowych oraz stylu życia na funkcję bariery jelitowej.
Introduction and objective:
Intestinal barrier dysfunction may promote increased migration of antigens, bacteria and toxins from the intestinal lumen into the bloodstream. This condition is observed in the course of various diseases, both acute and chronic. Modifying the intestinal barrier function could potentially affect the course of these diseases and patient prognosis. The aim of this article was to assess the role of selected nutritional components and lifestyle on human intestinal barrier function.
Review methods:
A literature review was conducted using information from the PubMed/MEDLINE and ScienceDirect databases published before January 2021.
Brief description of the state of knowledge:
Diet and lifestyle are basic environmental factors that affect the intestinal barrier function. Adequate intake of dietary fibre and zinc is necessary to maintain proper barrier function. Glutamine is a particularly important amino acid in the maintenance of the epithelial barrier of the gastrointestinal tract, and the demand for it significantly increases in the course of various clinical conditions. A high-fat diet and alcohol can disrupt the structure of the intestinal barrier, which can have negative health effects. Gluten has a specific effect on the intestinal barrier permeability through the ability to release zonulin; however, under physiological conditions this process is strictly controlled and probably not clinically significant in healthy people. Lifestyle factors, such as mental stress and intense exercise, may disrupt the intestinal barrier function, contributing to an increase in intestinal permeability.
Summary:
The available literature undoubtedly suggests a significant influence of many nutrients and lifestyle on the intestinal barrier function.
Intestinal barrier dysfunction may promote increased migration of antigens, bacteria and toxins from the intestinal lumen into the bloodstream. This condition is observed in the course of various diseases, both acute and chronic. Modifying the intestinal barrier function could potentially affect the course of these diseases and patient prognosis. The aim of this article was to assess the role of selected nutritional components and lifestyle on human intestinal barrier function.
Review methods:
A literature review was conducted using information from the PubMed/MEDLINE and ScienceDirect databases published before January 2021.
Brief description of the state of knowledge:
Diet and lifestyle are basic environmental factors that affect the intestinal barrier function. Adequate intake of dietary fibre and zinc is necessary to maintain proper barrier function. Glutamine is a particularly important amino acid in the maintenance of the epithelial barrier of the gastrointestinal tract, and the demand for it significantly increases in the course of various clinical conditions. A high-fat diet and alcohol can disrupt the structure of the intestinal barrier, which can have negative health effects. Gluten has a specific effect on the intestinal barrier permeability through the ability to release zonulin; however, under physiological conditions this process is strictly controlled and probably not clinically significant in healthy people. Lifestyle factors, such as mental stress and intense exercise, may disrupt the intestinal barrier function, contributing to an increase in intestinal permeability.
Summary:
The available literature undoubtedly suggests a significant influence of many nutrients and lifestyle on the intestinal barrier function.
Andrulewicz M. Wpływ wybranych składników pokarmowych oraz stylu życia na funkcję bariery jelitowej człowieka. Med Og Nauk Zdr. Doi: 10.26444/monz/139615
REFERENCJE (81)
1.
Ding RX, Goh WR, Wu RN, et al. Revisit gut microbiota and its impact on human health and disease. J Food Drug Anal. 2019; 27(3): 623–631. https://doi.org/10.1016/j.jfda....
2.
Mohajeri MH, Grummer RJM, Rastall RA, et al. The role of the mic-robiome for human health: from basic science to clinical applications. Eur J Nutr. 2018; 57(1): 1–14. https://doi.org/10.1007/s00394....
3.
Nie P, Li Z, Wang Y, et al. Gut microbiome interventions in human health and diseases. Med Res Rev. 2019; 39(6): 2286–2313. https://doi.org/10.1002/med.21....
4.
Vancamelbeke M, Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. 2017; 11(9): 821–834. https://doi.org/10.1080/174741....
5.
Lee SH. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res. 2015; 13(1): 11–8. https://doi.org/10.5217/ir.201....
6.
Mu Q, Kirby J, Reilly CM, et al. Leaky Gut As a Danger Signal for Auto-immune Diseases. Fron Immunol. 2017; 8: 598. https://doi.org/10.3389/fimmu.....
7.
Otani S, Coopersmith CM. Gut integrity in critical illness. J Intensive Care. 2019; 7: 17. https://doi.org/10.1186/s40560....
8.
Welsh FK, Farmery SM, Mac Lennan K. Gut barrier function in mal-nourished patients. Gut. 1998; 42(3): 396–401. https://doi.org/10.1136/gut.42....
9.
Bischoff SC, Barbara G, Buurman W, et al. Intestinal permeability – a new target for disease prevention and therapy. BMC Gastroenterol. 2014; 14: 189. https://dx.doi.org/10.1186%2Fs....
10.
Groschwitz KR, Hogan SP. Intestinal Barrier Function: Molecular Regulation and Disease Pathogenesis. J Allergy Clin Immunol. 2009; 124(1): 3–22. https://dx.doi.org/10.1016%2Fj....
11.
Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nu-trients. 2013; 5(4): 1417–35. https://doi.org/10.3390/nu5041....
12.
Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the de-leterious consequences of a diet deficient in microbiota-accessible car-bohydrates. Cell Metab. 2014; 20(5): 779–786. https://doi.org/10.1016/j.cmet....
13.
Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017; 8(2): 172–184. https://doi.org/10.1080/194909....
14.
Koh A, Vadder FD, Kovatcheva-Datchary P, et al. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016; 165(6): 1332–1345. https://doi.org/10.1016/j.cell....
15.
den Besten G, van Eunen K, Groen AK, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013; 54(9): 2325–2340. https://dx.doi.org/10.1194%2Fj....
16.
Camilleri M, Lyle BJ, Madsen KL, et al. Role for diet in normal gut barrier function: developing guidance within the framework of food--labeling regulations. Am J Physiol Gastrointest Liver Physiol. 2019; 317(1): G17-G39. https://doi.org/10.1152/ajpgi.....
17.
Van Herreweghen F, De Paepe K, Rourne H, et al. Mucin degradation niche as a driver of microbiome composition and Akkermansia muci-niphila abundance in a dynamic gut model is donor independent. FEMS Microbiol Ecol. 2018; 94(12). https://doi.org/10.1093/femsec....
18.
Desai MS, Seekatz AM, Koropatkin NM, et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhan-ces Pathogen Susceptibility. Cell. 2016; 167(5): 1339–1353. https://doi.org/10.1016/j.cell....
19.
Earle KA, Billings G, Sigal M, et al. Quantitative Imaging of Gut Mic-robiota Spatial Organization. Cell Host Microbe. 2015; 18(4): 478–488. https://doi.org/10.1016/j.chom....
20.
Johansson MEV, Gustafsson JK, Holmen-Larsson J, et al. Bacteria.penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014; 63(2): 281–91. https://doi.org/10.1136/gutjnl....
21.
Russo F, Linsalata M, Clemente C, et al. Inulin-enriched pasta improves intestinal permeability and modifies the circulating levels of zonulin and glucagon-like peptide 2 in healthy young volunteers. Nutr Res. 2012; 32(12): 940–6. https://doi.org/10.1016/j.nutr....
22.
Krawczyk M, Maciejewska D, Ryterska K, et al. Gut Permeability Might be Improved by Dietary Fiber in Individuals with Nonalcoholic Fatty Liver Disease (NAFLD) Undergoing Weight Reduction. Nutrients. 2018; 10(11): 1793. https://doi.org/10.3390/nu1011....
23.
Tap J, Furet JP, Bensaada M, et al. Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environ Microbiol. 2015; 17(12): 4954–64. https://doi.org/10.1111/1462-2....
24.
Mokkala K, Röytiö H, Munukka E, et al. Gut Microbiota Richness and Composition and Dietary Intake of Overweight Pregnant Women Are Re-lated to Serum Zonulin Concentration, a Marker for Intestinal Permeabi-lity. J Nutr. 2016; 146(9): 1694–700. https://doi.org/10.3945/jn.116....
25.
Murphy EA, Velazquez KT, Herbert KM. Influence of High-Fat-Diet on Gut Microbiota: A Driving Force for Chronic Disease Risk. Nurr Opin Clin Nutr Metab Care. 2015; 18(5): 515–520. https://dx.doi.org/10.1097%2FM....
26.
Duan Y, Zeng L, Zheng C, et al. Inflammatory Links Between High Fat Diets and Diseases. Front Immunol. 2018; 13(9): 2649. https://doi.org/10.3389/fimmu.....
27.
Appleby RN, Walters JRF. The role of bile acids in functional GI di-sorders. Neurogastroenterol Motil. 2014; 26(8): 1057–69. https://doi.org/10.1111/nmo.12....
28.
Rohr MW, Narasimhulu CA, Rudeski-Rohr TA, et al. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv Nutr. 2020; 11(1): 77–91. https://doi.org/10.1093/advanc....
29.
Stenman LK, Holma R, Korpela R. High-fat-induced intestinal per-meability dysfunction associated with altered fecal bile acids. World J Gastroenterol. 2012; 18(9): 923–9. https://doi.org/10.3748/wjg.v1....
30.
Guo X, Li J, Tang R, et al. High Fat Diet Alters Gut Microbiota and the Expression of Paneth Cell-Antimicrobial Peptides Preceding Changes of Circulating Inflammatory Cytokines. Mediators Inflamm. 2017; 2017: 9474896. https://dx.doi.org/10.1155%2F2....
31.
Rao RK, Samak G. Role of Glutamine in Protection of Intestinal Epi-thelial Tight Junctions. J Epithel Biol Pharmacol. 2012; 5: 47–54. https://dx.doi.org/10.2174%2F1....
32.
Stehle P, Kuhn KS. Glutamine: An Obligatory Parenteral Nutrition Substrate in Critical Care Therapy. Biomed Res Int. 2015; 545467. https://dx.doi.org/10.1155%2F2....
33.
Kim MH, Kim H. The Roles of Glutamine in the Intestine and Its Im-plication in Intestinal Diseases. Int J Mol Sci. 2017; 18(5): 1051. https://doi.org/10.3390/ijms18....
34.
Akagi R, Ohno M, Matsubara K, et al. Glutamine protects intestinal barrier function of colon epithelial cells from ethanol by modulating Hsp70 expression. Pharmacology. 2013; 91(1–2): 104–11. https://doi.org/10.1159/000345....
35.
De-Souza DA, Greene LJ. Intestinal permeability and systemic infec-tions in critically ill patients: effect of glutamine. Crit Care Med. 2005; 33(5): 1125–1135. https://doi.org/10.1097/01.ccm....
36.
Bollhalder L, Pfeil AM, Tomonaga Y, et al. A systematic literature review and meta-analysis of randomized clinical trials of parenteral glutamine supplementation. Clin Nutr. 2013; 32(2): 213–223. https://doi.org/10.1016/j.clnu....
37.
Tao KM, Li XQ, Yang LQ, et al. Glutamine supplementation for critically ill adults. Cochrane Database Syst Rev. 2014; 9: CD010050. https://doi.org/10.1002/146518....
38.
Shariatpanahi ZV, Eslamian G, Ardehali SH. Effects of Early Enteral Glutamine Supplementation on Intestinal Permeability in Critically Ill Patients. Indian J Crit Care Med. 2019; 23(8): 356–362. https://dx.doi.org/10.5005%2Fj....
39.
Garcia-de-Lorenzo A, Zarazaga A, Garcia Luna PP, et al. Clinical evidence for enteral nutritional support with glutamine: a systematic review. Nutrition. 2003; 19(9): 805–811. https://doi.org/10.1016/s0899-....
40.
Shu XL, Yu TT, Kang K, et al. Effects of glutamine on markers of intestinal inflammatory response and mucosal permeability in abdo-minal surgery patients: A meta-analysis. Exp Ther Med. 2016; 12(6): 3499–3506. https://dx.doi.org/10.3892%2Fe....
41.
Hond ED, Peeters M, Hiele M, et al. Effect of glutamine on the intestinal permeability changes induced by indomethacin in humans. Aliment Pharmacol Ther. 1999; 13(5): 679–685. https://doi.org/10.1046/j.1365....
42.
Pugh JN, Sage S, Hutson M, et al. Glutamine supplementation redu-ces markers of intestinal permeability during running in the heat in a dose-dependent manner. Eur J Appl Physiol. 2017; 117(12): 2569–2577. https://doi.org/10.1007/s00421....
43.
Zuhl MN, Lanphere KR, Kravitz L, et al. Effects of oral glutamine su-pplementation on exercise-induced gastrointestinal permeability and tight junction protein expression. J Appl Physiol. 2014; 116(2): 183–191. https://doi.org/10.1152/japplp....
44.
Zhou Q, Verne ML, Fields JZ, et al. Randomised placebo-controlled trial of dietary glutamine supplements for postinfectious irritable bowel syndro-me. Gut. 2019; 68(6): 996–1002. https://doi.org/10.1136/gutjnl....
45.
Ohashi W, Fukada T. Contribution of Zinc and Zinc Transporters in the Pathogenesis of Inflammatory Bowel Diseases. J Immunol Res. 2019; 8396878. https://doi.org/10.1155/2019/8....
46.
Lamberti LM, Walker CL, Chan KY, et al. Oral zinc supplementation for the treatment of acute diarrhea in children: a systematic review and meta--analysis. Nutrients. 2013; 5(11): 4715–40. https://doi.org/10.3390/nu5114....
47.
Alam AN, Sarker SA, Wahed MA, et al. Enteric protein loss and intesti-nal permeability changes in children during acute shigellosis and after recovery: effect of zinc supplementation. Gut. 1994; 35(12): 1707–11. https://doi.org/10.1136/gut.35....
48.
Sturniolo GC, Di Leo V Ferronato A, D‘Odorico A, et al. Zinc supple-mentation tightens „leaky gut” in Crohn's disease. Inflamm Bowel Dis. 2001; 7(2): 94–8. https://doi.org/10.1097/000547....
49.
Hewlings S, Kalman D. A Review of Zinc-L-Carnosine and Its Positive Ef-fects on Oral Mucositis, Taste Disorders, and Gastrointestinal Disorders. Nutrients. 2020; 12(3): 665. https://dx.doi.org/10.3390%2Fn....
50.
Mahmood A, FitzHerald AJ, Marchbank T, et al. Zinc carnosine, a he-alth food supplement that stabilises small bowel integrity and stimu-lates gut repair processes. Gut. 2007; 56(2): 168–175. https://dx.doi.org/10.1136%2Fg....
51.
Davison G, Marchbank T, March DS, et al. Zinc carnosine works with bovine colostrum in truncating heavy exercise-induced increase in gut permeability in healthy volunteers. Am J Clin Nutr. 2016; 104(2): 526–536. https://doi.org/10.3945/ajcn.1....
52.
Miyoshi Y, Tanabe S, Suzuki T. Cellular zinc is required for intestinal epithelial barrier maintenance via the regulation of claudin-3 and oc-cludin expression. Am J Physiol Gastrointest Liver Physiol. 2016; 311(1): G105–16. https://doi.org/10.1152/ajpgi.....
53.
Wang X, Valenzano MC, Mercado JM, et al. Zinc supplementation modifies tight junctions and alters barrier function of CACO-2 human intestinal epithelial layers. Dig Dis Sci. 2013; 58(1): 77–87. https://doi.org/10.1007/s10620....
54.
Mocan O, Dumitrascu DL. The broad spectrum of celiac disease and gluten sensitive enteropathy. Clujul Med. 2016; 89(3): 335–342. https://dx.doi.org/10.15386%2F....
55.
Gutiérrez S, Pérez-Andrés J, Martinez-Blanco H, et al. The human digestive tract has proteases capable of gluten hydrolysis. Mol Metab. 2017; 6(7): 693–702. https://dx.doi.org/10.1016%2Fj....
56.
Fasano A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res. 2020; 9: F1000 Faculty Rev-69. https://dx.doi.org/10.12688%2F....
57.
Asmar RE, Panigrahi P, Bamford P, et al. Host-dependent zonulin secre-tion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology. 2002; 123(5): 1607–15. https://doi.org/10.1053/gast.2....
58.
Niland B, Cash BD. Health Benefits and Adverse Effects of a Gluten--Free Diet in Non–Celiac Disease Patients. Gastroenterol Hepatol. 2018; 14(2): 82–91.
59.
Drago S, Asmar RE, Di Pieroo M, et al. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol. 2006; 41(4): 408–19. https://doi.org/10.1080/003655....
60.
Hollon J, Puppa EL, Greenwald B, et al. Effect of gliadin on permeability of intestinal biopsy explants from celiac disease patients and patients with non-celiac gluten sensitivity. Nutrients 2015; 7(3): 1565–76. https://doi.org/10.3390/nu7031....
61.
Lerner A, Shoenfeld Y, Matthias T. Adverse effects of gluten ingestion and advantages of gluten withdrawal in nonceliac autoimmune disease. Nutr Rev. 2017; 75(12): 1046–1058. https://doi.org/10.1093/nutrit....
62.
Ratna A, Mandrekar P. Alcohol and Cancer: Mechanisms and Thera-pies. Biomolecules. 2017; 7(3): 61. https://doi.org/10.3390/biom70....
63.
Mathurin P, Bataller R. Trends in the management and burden of alcoholic liver disease. J Hepatol. 2015; 62(1): S38–46. https://doi.org/10.1016/j.jhep....
64.
Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet. 1984; 1(8370): 179–82. https://doi.org/10.1016/s0140-....
65.
Wang Y, Tong J, Chang B, et al. Effects of alcohol on intestinal epithe-lial barrier permeability and expression of tight junction-associated proteins. Mol Med Rep. 2014; 9(6): 2352–6. https://doi.org/10.3892/mmr.20....
66.
Elamin E, Masclee A, Troost F, et al. Ethanol Impairs Intestinal Bar-rier Function in Humans through Mitogen Activated Protein Kinase Signaling: A Combined In Vivo and In Vitro Approach. PLoS One. 2014; 9(9): e107421. https://dx.doi.org/10.1371%2Fj....
67.
Parlesak A, Schäfer C, Schütz T, et al. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000; 32(5): 742–7. https://doi.org/10.1016/s0168-....
68.
Swanson GR, Tieu V, Shaikh M, et al. Is moderate red wine consumption safe in inactive inflammatory bowel disease? Digestion. 2011; 84(3): 238–44. https://doi.org/10.1159/000329....
69.
Elamin EE, Masclee AA, Dekker J, et al. Ethanol metabolism and its effects on the intestinal epithelial barrier. Nutr Rev. 2013; 71(7): 483–99. https://doi.org/10.1111/nure.1....
70.
Bishehsari F, Magno E, Swanson G. et al. Alcohol and Gut-Derived Inflammation. Alcohol Res. 2017; 38(2): 163–171.
71.
Rampelli S, Candela M, Turroni S, et al. Microbiota and lifestyle inte-ractions through the lifespan. Trends in Food Science & Technology. 2016; 57(B): 265–272.
72.
Keita AV, Söderholm JD, Ericson AC. Stress-induced barrier disrup-tion of rat follicle-associated epithelium involves corticotropin-relea-sing hormone, acetylcholine, substance P, and mast cells. Neuroga-stroenterol Motil. 2010; 22(7): 770–8. https://doi.org/10.1111/j.1365....
73.
Vicario M, Guilarte M, Alonso C, et al. Chronological assessment of mast cell-mediated gut dysfunction and mucosal inflammation in a rat model of chronic psychosocial stress. Brain Behav Immun. 2010; 24(7): 1166–75. https://doi.org/10.1016/j.bbi.....
74.
Vanuytsel T, van Wanrooy S, Vanheel H, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014; 63(8): 1293–9. https://doi.org/10.1136/gutjnl....
75.
de Oliveira EP, Burini RC, Jeukendrup A. Gastrointestinal Com-plaints During Exercise: Prevalence, Etiology, and Nutritional Re-commendations. Sports Med. 2014; 44(1): 79–85. https://dx.doi.org/10.1007%2Fs....
76.
Pugh JN, Fearn R, Morton JP, et al. Gastrointestinal symptoms in elite athletes: time to recognise the problem? Br J Sports Med. 2018; 52(8): 487–488. https://doi.org/10.1136/bjspor....
77.
Joyner MJ, Dasey DP. Regulation of Increased Blood Flow (Hyperemia) to Muscles During Exercise: A Hierarchy of Competing Physiological Needs. Physiol Rev. 2015; 95(2): 549–601. https://dx.doi.org/10.1152%2Fp....
78.
van Wijck K, Lenaerts K, Grootjans J, et al. Physiology and pathophysio-logy of splanchnic hypoperfusion and intestinal injury during exercise: strategies for evaluation and prevention. Am J Physiol Gastrointest Liver Physiol. 2012; 303(2): G155–68. https://doi.org/10.1152/ajpgi.....
79.
Chantler S, Griffiths A, Matu J, et al. The Effects of Exercise on Indirect Markers of Gut Damage and Permeability: A Systematic Review and Meta-analysis. Sports Med. 2021; 51(1): 113–124. https://doi.org/10.1007/s40279....
80.
Li X, Kan EM, Lu J, et al. Combat-training increases intestinal perme-ability, immune activation and gastrointestinal symptoms in soldiers. Aliment Pharmacol Ther. 2013; 37(8): 799–809. https://doi.org/10.1111/apt.12....
81.
Marchbank T, Davison G, Oakes JR, et al. The nutriceutical bovine colostrum truncates the increase in gut permeability caused by heavy exercise in athletes. Am J Physiol Gastrointest Liver Physiol. 2011; 300(3): G477–84. https://doi.org/10.1152/ajpgi.....
Udostępnij
ARTYKUŁ POWIĄZANY
Przetwarzamy dane osobowe zbierane podczas odwiedzania serwisu. Realizacja funkcji pozyskiwania informacji o użytkownikach i ich zachowaniu odbywa się poprzez dobrowolnie wprowadzone w formularzach informacje oraz zapisywanie w urządzeniach końcowych plików cookies (tzw. ciasteczka). Dane, w tym pliki cookies, wykorzystywane są w celu realizacji usług, zapewnienia wygodnego korzystania ze strony oraz w celu monitorowania ruchu zgodnie z Polityką prywatności. Dane są także zbierane i przetwarzane przez narzędzie Google Analytics (więcej).
Możesz zmienić ustawienia cookies w swojej przeglądarce. Ograniczenie stosowania plików cookies w konfiguracji przeglądarki może wpłynąć na niektóre funkcjonalności dostępne na stronie.
Możesz zmienić ustawienia cookies w swojej przeglądarce. Ograniczenie stosowania plików cookies w konfiguracji przeglądarki może wpłynąć na niektóre funkcjonalności dostępne na stronie.