Online first
Bieżący numer
Archiwum
O czasopiśmie
Polityka etyki publikacyjnej
System antyplagiatowy
Instrukcje dla Autorów
Instrukcje dla Recenzentów
Rada Redakcyjna
Komitet Redakcyjny
Recenzenci
Wszyscy recenzenci
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
Kontakt
Bazy indeksacyjne
Klauzula przetwarzania danych osobowych (RODO)
PRACA ORYGINALNA
Badania wstępne nad występowaniem pierwotniaków z rodzaju Theileria u kleszczy we wschodniej Polsce
1
Zakład Biologicznych Szkodliwości Zdrowotnych i Parazytologii, Instytut Medycyny Wsi im. Witolda Chodźki, Lublin, Polska
Autor do korespondencji
Anna Kloc
Zakład Biologicznych Szkodliwości Zdrowotnych i Parazytologii, Instytut Medycyny Wsi im. Witolda Chodźki, Lublin, Polska, Jaczewskiego 2, 20-090, Lublin, Polska
Zakład Biologicznych Szkodliwości Zdrowotnych i Parazytologii, Instytut Medycyny Wsi im. Witolda Chodźki, Lublin, Polska, Jaczewskiego 2, 20-090, Lublin, Polska
Med Og Nauk Zdr. 2021;27(4):441-447
SŁOWA KLUCZOWE
DZIEDZINY
STRESZCZENIE
Wprowadzenie i cel:
Pierwotniaki z rodzaju Theileria przenoszone przez kleszcze wywołują ciężkie jednostki chorobowe u zwierząt, głównie przeżuwaczy. Celem badań była ocena występowania pierwotniaków Theileria spp. u kleszczy z gatunku Ixodes ricinus i Dermacentor reticulatus pozyskanych na terenie Lubelszczyzny.
Materiał i metody:
Materiał do badań stanowiła grupa 30 napitych samic kleszczy zdjętych z ciał żywicieli. Samice w warunkach laboratoryjnych złożyły jaja, z części z nich wylęgły się larwy. Wyizolowany materiał genetyczny z samic, jaj i larw został poddany analizie przy użyciu łańcuchowej reakcji polimerazy (PCR) z wykorzystaniem primerów specyficznych względem pierwotniaków z rodzaju Theileria.
Wyniki:
W grupie 30 samic kleszczy oznaczono 15 samic z gatunku Ixodes ricinus i 15 samic z gatunku Dermacentor reticulatus. Łącznie samice złożyły 6720 jaj, przy czym z 2440 jaj wykluły się larwy (36,31%). Zarówno jaja, jak i larwy zostały przebadane w pulach po 20 sztuk. W badanym materiale DNA wyizolowanym z samic, jaj i larw nie wykryto pierwotniaków Theileria spp.
Wnioski:
Jak dotąd nieliczne badania dotyczyły występowania pierwotniaków Theileria spp. w krajowych kleszczach, dlatego podjęte badania miały na celu dostarczyć nowych informacji na ten temat. Nie stwierdzono obecności ww. pierwotniaków w materiale genetycznym wyizolowanym z samic, jaj i larw kleszczy z gatunku I. ricinus i D. reticulatus. Uniemożliwiło to wnioskowanie na temat potencjalnej roli transmisji transowarialnej jako drogi szerzenia się Theileria spp. w środowisku. Podsumowując, uzyskane wyniki nie potwierdziły udziału powszechnie występujących w Polsce kleszczy w przenoszeniu Theileria spp.
Pierwotniaki z rodzaju Theileria przenoszone przez kleszcze wywołują ciężkie jednostki chorobowe u zwierząt, głównie przeżuwaczy. Celem badań była ocena występowania pierwotniaków Theileria spp. u kleszczy z gatunku Ixodes ricinus i Dermacentor reticulatus pozyskanych na terenie Lubelszczyzny.
Materiał i metody:
Materiał do badań stanowiła grupa 30 napitych samic kleszczy zdjętych z ciał żywicieli. Samice w warunkach laboratoryjnych złożyły jaja, z części z nich wylęgły się larwy. Wyizolowany materiał genetyczny z samic, jaj i larw został poddany analizie przy użyciu łańcuchowej reakcji polimerazy (PCR) z wykorzystaniem primerów specyficznych względem pierwotniaków z rodzaju Theileria.
Wyniki:
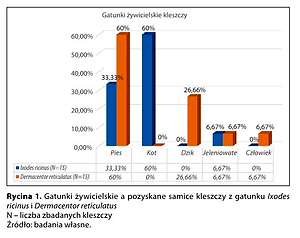
W grupie 30 samic kleszczy oznaczono 15 samic z gatunku Ixodes ricinus i 15 samic z gatunku Dermacentor reticulatus. Łącznie samice złożyły 6720 jaj, przy czym z 2440 jaj wykluły się larwy (36,31%). Zarówno jaja, jak i larwy zostały przebadane w pulach po 20 sztuk. W badanym materiale DNA wyizolowanym z samic, jaj i larw nie wykryto pierwotniaków Theileria spp.
Wnioski:
Jak dotąd nieliczne badania dotyczyły występowania pierwotniaków Theileria spp. w krajowych kleszczach, dlatego podjęte badania miały na celu dostarczyć nowych informacji na ten temat. Nie stwierdzono obecności ww. pierwotniaków w materiale genetycznym wyizolowanym z samic, jaj i larw kleszczy z gatunku I. ricinus i D. reticulatus. Uniemożliwiło to wnioskowanie na temat potencjalnej roli transmisji transowarialnej jako drogi szerzenia się Theileria spp. w środowisku. Podsumowując, uzyskane wyniki nie potwierdziły udziału powszechnie występujących w Polsce kleszczy w przenoszeniu Theileria spp.
Introduction and objective:
Protozoa of the genus Theileria transmitted by ticks cause a severe disease in animals, mainly ruminants. The aim of the project was to investigate the occurrence of protozoa Theileria spp. in Ixodes ricinus and Dermacentor reticulatus species using molecular biology methods in the Lublin Province.
Material and methods:
The research material was a group of 30 engorged female ticks removed from the host bodies. In laboratory conditions, the females laid eggs out of which some of the larvae emerged. Then, the isolated genetic material from females, eggs and larvae was analyzed by PCR using primers specific for the protozoa of the genus Theileria spp.
Results:
In the study group of 30 female ticks, 15 females of the species Ixodes ricinus and 15 females of the species Dermacentor reticulatus were identified. In total, females laid 6,720 eggs, of which 2,440 larvae hatched (36.31%). Both eggs and larvae were tested in pools of 20. Theileria protozoa were detected neither in the tested DNA material isolated from females nor in egg and larvae isolates.
Conclusions:
In Poland, there are few studies on Theileria spp. The tests for Theileria spp. showed negative results in the genetic material of females, eggs and larvae in both species of ticks. This made it impossible to draw conclusions about the potential role of transovarial transmission as a pathway for the spread of Theileria spp. in the environment. Summing up, the obtained results did not confirm the participation of the common ticks in the transmission of Theileria spp.
Protozoa of the genus Theileria transmitted by ticks cause a severe disease in animals, mainly ruminants. The aim of the project was to investigate the occurrence of protozoa Theileria spp. in Ixodes ricinus and Dermacentor reticulatus species using molecular biology methods in the Lublin Province.
Material and methods:
The research material was a group of 30 engorged female ticks removed from the host bodies. In laboratory conditions, the females laid eggs out of which some of the larvae emerged. Then, the isolated genetic material from females, eggs and larvae was analyzed by PCR using primers specific for the protozoa of the genus Theileria spp.
Results:
In the study group of 30 female ticks, 15 females of the species Ixodes ricinus and 15 females of the species Dermacentor reticulatus were identified. In total, females laid 6,720 eggs, of which 2,440 larvae hatched (36.31%). Both eggs and larvae were tested in pools of 20. Theileria protozoa were detected neither in the tested DNA material isolated from females nor in egg and larvae isolates.
Conclusions:
In Poland, there are few studies on Theileria spp. The tests for Theileria spp. showed negative results in the genetic material of females, eggs and larvae in both species of ticks. This made it impossible to draw conclusions about the potential role of transovarial transmission as a pathway for the spread of Theileria spp. in the environment. Summing up, the obtained results did not confirm the participation of the common ticks in the transmission of Theileria spp.
Kloc A. Badania wstępne nad występowaniem pierwotniaków z rodzaju Theileria u kleszczy we wschodniej Polsce. Med Og Nauk Zdr. 2021;
27(4): 441–447. doi: 10.26444/monz/145060
REFERENCJE (50)
1.
Adamska M, Skotarczak B. Molecular detecting of piroplasms in feeding and questing Ixodes ricinus ticks.Ann Parasitol. 2017; 63(1): 21–26. https://doi: 10.17420/ap6301.80.
2.
Mans BJ, Pienaar R, Latif AA. A review of Theileria diagnostics and epidemiology. Int J Parasitol Parasites Wildl. 2015; 6; 4(1): 104–18. https://doi: 10.1016/j.ijppaw.2014.12.006.
3.
Wiens O, Xia D, von Schubert C, Wastling JM, Dobbelaere DA, Heussler VT, Woods KL. Cell cycle-dependent phosphorylation of Theileria annulata schizont surface proteins. PLoS One. 2014; 31; 9(7): e103821. https://doi: 10.1371/journal.pone.0103821.
4.
Kabi F, Masembe C, Muwanika V, Kirunda H, Negrini R. Geographic distribution of non-clinical Theileria parva infection among indigenous cattle populations in contrasting agro-ecological zones of Uganda: implications for control strategies. Parasit Vectors. 2014; 1; 7: 414. https://doi: 10.1186/1756-3305-7-414.
5.
Kazungu YE, Mwega E, Neselle MO, Sallu R, Kimera SI, Gwakisa P. Incremental effect of natural tick challenge on the infection and treatment method-induced immunity against T. parva in cattle under agro-pastoral systems in Northern Tanzania. Ticks Tick Borne Dis. 2015; 6(5): 587–91. https://doi: 10.1016/j.ttbdis.2015.04.014.
6.
Baptista C, Lopes MS, Tavares AC, Rojer H, Kappmeyer L, Mendonça D, da Câmara Machado A. Diagnosis of Theileria equi infections in horses in the Azores using cELISA and nested PCR. Ticks Tick Borne Dis. 2013; 4(3): 242–5. https://doi: 10.1016/j.ttbdis.2012.11.008.
7.
Aydin MF, Aktas M, Dumanli N. Molecular identification of Theileria and Babesia in ticks collected from sheep and goats in the Black Sea region of Turkey. Parasitol Res. 2015; 114(1): 65–9. https://doi: 10.1007/s00436-014-4160-x.
8.
Sivakumar T, Hayashida K, Sugimoto C, Yokoyama N. Evolution and genetic diversity of Theileria. Infect Genet Evol. 2014; 27: 250–63. https://doi: 10.1016/j.meegid.2014.07.013.
9.
Sumrandee C, Baimai V, Trinachartvanit W, Ahantarig A. Hepatozoon and Theileria species detected in ticks collected from mammals and snakes in Thailand. Ticks Tick Borne Dis. 2015; 6(3): 309–15. https://doi: 10.1016/j.ttbdis.2015.02.003.
10.
Mohammad Al-Saeed AT, Omer LT, Abdo J, Habibi G, Salih DA, Seitzer U, Ahmed J. Epidemiological studies on tropical theileriosis (Theileria annulata infection of cattle) in Kurdistan Region, Iraq. Parasitol Res. 2010; 106(2): 403–7. https://doi: 10.1007/s00436-009-1675-7.
11.
De Goeyse I, Jansen F, Madder M, Hayashida K, Berkvens D, Dobbe-laere D, Geysen D. Transfection of live, tick derived sporozoites of the protozoan Apicomplexan parasite Theileria parva. Vet Parasitol. 2015; 15; 208(3–4): 238–41. https://doi: 10.1016/j.vetpar.2015.01.013.
12.
Cayir E, Erdemir A, Ozkan E, Topuzogullari M, Bolat ZB, Akat A, Turgut-Balik D. Cloning of intron-removed enolase gene and expression, purification, kinetic characterization of the enzyme from Theileria annulata. Mol Biotechnol. 2014; 56(8): 689–96. https://doi: 10.1007/s12033-014-9747-z.
13.
Githaka N, Konnai S, Bishop R, Odongo D, Lekolool I, Kariuki E, Gakuya F, Kamau L, Isezaki M, Murata S, Ohashi K. Identification and sequence characterization of novel Theileria genotypes from the waterbuck (Kobus defassa) in a Theileria parva-endemic area in Kenya. Vet Parasitol. 2014; 28; 202(3–4): 180–93. https://doi: 10.1016/j.vetpar.2014.02.056.
14.
Obara I, Ulrike S, Musoke T, Spooner PR, Jabbar A, Odongo D, Kemp S, Silv JC Bishop RP. Molecular evolution of a central region containing B cell epitopes in the gene encoding the p67 sporozoite antigen within a field population of Theileria parva. Parasitol Res. 2015; 114(5): 1729–37. https://doi: 10.1007/s00436-015-4358-6.
15.
Alanazi AD, Said AE, Morin-Adeline V, Alyousif MS, Slapeta J. Quantitative PCR detection of Theileria equi using laboratory workflows to detect asymptomatic persistently infected horses. Vet Parasitol. 2014; 15; 206(3–4): 138–45. https://doi: 10.1016/j.vetpar.2014.09.019.
16.
Hammer JF, Emery D, Bogema DR, Jenkins C. Detection of Theileria orientalis genotypes in Haemaphysalis longicornis ticks from southern Australia. Parasit Vectors. 2015; 16; 8(1): 229. https://doi: 10.1186/s13071-015-0839-9.
17.
Jalali SM, Khaki Z, Kazemi B, Rahbari S, Shayan P, Bandehpour M, Yasini SP. Molecular Detection and Identification of Theileria Species by PCR-RFLP Method in Sheep from Ahvaz, Southern Iran. Iran J Parasitol. 2014; 9(1): 99–106.
18.
Njuguna JT, von Koschitzky I, Gerhardt H, Lämmerhofer M, Choucry A, Pink M, Schmitz-Spahnke S, Bakheit MA, Strube C, Kaiser A. Target evaluation of deoxyhypusine synthase from Theileria parva the neglected animal parasite and its relationship to Plasmodium. Bioorg Med Chem. 2014; 1;22(15): 4338–46. https://doi: 10.1016/j.bmc.2014.05.007.
19.
Skotarczak B. Biologia molekularna patogenów przenoszonych przez kleszcze. Redakcja naukowa. Warszawa: Wydawnictwo lekarskie PZWL; 2006.
20.
Perera PK, Gasser RB, Jabbar A. Assessment of sequence variability in a p23 gene region within and among three genotypes of the Theileria orientalis complex from south-eastern Australia. Ticks Tick Borne Dis. 2015; 6(2): 123–8. https://doi: 10.1016/j.ttbdis.2014.10.006.
21.
Ghaemi P, Hoghooghi-Rad N, Shayan P, Eckert B. Detection of Theile -ria orientalis in Iran by semi-nested PCR. Parasitol Res. 2012; 110(2): 527–31. https://doi: 10.1007/s00436-011-2517-y.
22.
Bawm S, Shimizu K, Hirota J, Tosa Y, Htun LL, Maw NN, Thein M, Kato H, Sakurai T, Katakura K. Molecular prevalence and genetic diversity of bovine Theileria orientalis in Myanmar. Parasitol Int. 2014; 63(4): 640–5. https://doi: 10.1016/j.parint.2014.04.009.
23.
Hines SA, Ramsay JD, Kappmeyer LS, Lau AO, Ojo KK, Van Voorhis WC, Knowles DP, Mealey RH. Theileria equi isolates vary in susceptibility to imidocarb dipropionate but demonstrate uniform in vitro susceptibility to a bumped kinase inhibitor. Parasit Vectors. 2015; 20; 8(1): 33. https://doi: 10.1186/s13071-014-0611-6.
24.
Laus F, Spaterna A, Faillace V, Veronesi F, Ravagnan S, Beribé F, Cerquetella M, Meligrana M, Tesei B. Clinical investigation on Theileria equi and Babesia caballi infections in Italian donkeys. BMC Vet Res. 2015; 28; 11(1): 100. https://doi: 10.1186/s12917-015-0411-z.
25.
Malekifard F, Tavassoli M, Yakhchali M, Darvishzadeh R. Detection of Theileria equi and Babesia caballi using microscopic and molecular methods in horses in suburb of Urmia, Iran. Vet Res Forum. 2014 Spring; 5(2): 129–33.
26.
Piantedosi D, D‘Alessio N, Di Loria A, Di Prisco F, Mariani U, Neola B, Santoro M, Montagnaro S, Capelli G, Veneziano V. Seroprevalence and risk factors associated with Babesia caballi and Theileria equi infections in donkeys from Southern Italy. Vet J. 2014; 202(3): 578–82. https://doi: 10.1016/j.tvjl.2014.09.025.
27.
Prochno HC, Scorsin LM, De Melo FR, Baldani CD, Falbo MK, de Aquino LC, Lemos KR. Seroprevalence rates of antibodies against Theileria equi in team roping horses from central-western region of Paraná. Rev Bras Parasitol Vet. 2014; 23(1): 85–9. https://doi: 10.1590/s1984-29612014012.
28.
Sawczuk M, Maciejewska A, Adamska M, Skotarczak B. [Roe deer (Capreolus capreolus) and red deer (Cervus elaphus) as a reservoir of protozoans from Babesia and Theileria genus in north-western Poland].Wiad Parazytol. 2005; 51(3): 243–7.
29.
Slivinska K, Víchová B, Werszko J, Szewczyk T, Wróblewski Z, Peťko B, Ragač O, Demeshkant V, Karbowiak G. Molecular surveillance of Theileria equi and Anaplasma phagocytophilum infections in horses from Ukraine, Poland and Slovakia. Vet Parasitol. 2016; 15; 215: 35–7. https://doi: 10.1016/j.vetpar.2015.10.025.
30.
Rijpkema S, Nieuwenhuijs J, Franssen FF, Jongejan F. Infection rates of Borrelia burgdorferi in different instars of Ixodes ricinus ticks from the Dutch North Sea Island of Ameland. Exp Appl Acarol. 1994; 18(9): 531–42. https://doi: 10.1007/BF00058936.
31.
Pesquera C, Portillo A, Palomar AM, Oteo JA. Investigation of tick-borne bacteria (Rickettsia spp., Anaplasma spp., Ehrlichia spp. and Borrelia spp.) in ticks collected from Andean tapirs, cattle and vegetation from a protected area in Ecuador. Parasit Vectors. 2015; 24; 8: 46. https://doi: 10.1186/s13071-015-0662-3.
32.
Li Y, Liu J, Liu Z, Yang J, Li Y, Li Q, Qin G, Chen Z, Guan G, Luo J, Yin H. Report of Theileria luwenshuni and Theileria sp. RSR from cervids in Gansu, China. Parasitol Res. 2015; 114(5): 2023–9. https://doi: 10.1007/s00436-015-4439-6.
33.
Zając V, Wójcik-Fatla A, Sawczyn A, Cisak E, Sroka J, Kloc A, Zając Z, Buczek A, Dutkiewicz J, Bartosik K. Prevalence of infections and co-infections with 6 pathogens in Dermacentor reticulatus ticks collected in eastern Poland. Ann Agric Environ Med. 2017; 21; 24(1): 26–32. https://doi: 10.5604/12321966.1233893.
34.
Wójcik-Fatla A, Cisak E, Zając V, Zwoliński J, Dutkiewicz J. Prevalence of tick-borne encephalitis virus in Ixodes ricinus and Dermacentor reticulatus ticks collected from the Lublin region (eastern Poland). Ticks Tick Borne Dis. 2011; 2(1): 16–9. https://doi: 10.1016/j.ttbdis.2010.10.001.
35.
Wójcik-Fatla A, Zając V, Sawczyn A, Sroka J, Cisak E, Dutkiewicz J. Infections and mixed infections with the selected species of Borrelia burgdorferi sensu lato complex in Ixodes ricinus ticks collected in eastern Poland: a significant increase in the course of 5 years. Exp Appl Acarol. 2016; 68(2): 197–212. https://doi: 10.1007/s10493-015-9990-4.
36.
Rollend L, Fish D, Childs JE. Transovarial transmission of Borrelia pirochetes by Ixodes scapularis: a summary of the literature and recent observations. Ticks Tick Borne Dis. 2013; 4(1–2): 46–51. https://doi: 10.1016/j.ttbdis.2012.06.008.
37.
Pańczuk A, Tokarska-Rodak M, Zarębska M, Pawłowicz-Sosnowska E. Species diversity of ticks infesting dogs in the north-eastern part of Lublin Province (eastern Poland). Ann Parasitol. 2021; 67(1): 79–83. https://doi: 10.17420/ap6701.314.
38.
Mierzejewska EJ, Welc-Faleciak R, Karbowiak G, Kowalec M, Behnke JM, Bajer A. Dominance of Dermacentor reticulatus over Ixodes ricinus (Ixodidae) on livestock, companion animals and wild ruminants in eastern and central Poland. Exp Appl Acarol. 2015; 66(1): 83–101. https://doi: 10.1007/s10493-015-9889-0.
39.
Muhanguzi D, Picozzi K, Hatendorf J, Thrusfield M, Welburn SC, Kabasa JD, Waiswa C. Collateral benefits of restricted insecticide application for control of African trypanosomiasis on Theileria parva in cattle: a randomized controlled trial. Parasit Vectors. 2014; 8; 7: 432. https://doi: 10.1186/1756-3305-7-432.
40.
Díaz-Cao JM, Adaszek Ł, Dzięgiel B, Paniagua J, Caballero-Gómez J, Winiarczyk S, Winiarczyk D, Cano-Terriza D, García-BocanegraI. Prevalence of selected tick-borne pathogens in wild ungulates and ticks in southern Spain. Transbound Emerg Dis. 2021; 8. https://doi: 10.1111/tbed.14065.
41.
Romiti F, Magliano A, Antognetti V, Manna G, Cersini A, Scicluna MT, De Liberato C. J Investigation of Ixodid ticks as vectors of Babesia caballi and Theileria equi (Protozoa: Apicomplexa) in central Italy. Vector Ecol. 2020; 45(1): 25–31. https://doi: 10.1111/jvec.12370.
42.
Hao L, Yuan D, Li S, Jia T, Guo L, Hou W, Lu Z, Mo X, Yin J, Yang A, Zheng W, Li R. Detection of Theileria spp. in ticks, sheep keds (Melophagus ovinus), and livestock in the eastern Tibetan Plateau, China. Parasitol Res. 2020; 119(8): 2641–2648. https://doi: 10.1007/s00436-020-06757-6.
43.
Slivinska K, Karbowiak G, Gawor J, Wróblewski Z, Jaworski Z, Jastrzębska E, Demeshkant V. Parasitic fauna of Polish konik horses (Equus caballus gmelini Antonius) and their impact on breeding: a review. Anim Health Res Rev. 2018; 19(2): 162–165. https://doi: 10.1017/S1466252318000099.
44.
Orkun Ö. Molecular investigation of the natural transovarial transmission of tick-borne pathogens in Turkey. Vet Parasitol. 2019; 273: 97–104. https://doi: 10.1016/j.vetpar.2019.08.013.
45.
Ghosh S, Azhahianambi P. Laboratory rearing of Theileria annulata-free Hyalomma anatolicum anatolicum ticks. Exp Appl Acarol. 2007; 43(2): 137–46. https://doi: 10.1007/s10493-007-9100-3.
46.
Uilenberg G. Babesia – a historical overview. Vet Parasitol. 2006; 31; 138(1–2): 3–10. https://doi: 10.1016/j.vetpar.2006.01.035.
47.
Adaszek Ł, Górna M, Krzysiak M, Adaszek M, Garbal M, Winiarczyk S. Identification of the piroplasms isolated from horses with clinical piroplasmosis in Poland. Wiad Parazytol. 2011; 57(1): 21–6.
48.
Dewangan P, Panigrahi M, Kumar A, Saravanan BC, Ghosh S, Asaf VN, Parida S, Gaur GK, Sharma D, Bhushan B. The mRNA expression of immune-related genes in crossbred and Tharparkar cattle in response to in vitro infection with Theileria annulata. Mol Biol Rep. 2015; 42(8): 1247–55. https://doi: 10.1007/s11033-015-3865-y.
49.
Echebli N, Mhadhbi M, Chaussepied M, Vayssettes C, Di Santo JP, Darghouth MA, Langsley G. Engineering attenuated virulence of a Theileria annulata-infected macrophage. PLoS Negl Trop Dis. 2014; 6; 8(11): e3183. https://doi: 10.1371/journal.pntd.0003183.
50.
Tajeri S, Langsley G. Theileria secretes proteins to subvert its host leukocyte. Biol Cell. 2021; 113(4): 220–233. https://doi: 10.1111/boc.202000096.
Udostępnij
ARTYKUŁ POWIĄZANY
Przetwarzamy dane osobowe zbierane podczas odwiedzania serwisu. Realizacja funkcji pozyskiwania informacji o użytkownikach i ich zachowaniu odbywa się poprzez dobrowolnie wprowadzone w formularzach informacje oraz zapisywanie w urządzeniach końcowych plików cookies (tzw. ciasteczka). Dane, w tym pliki cookies, wykorzystywane są w celu realizacji usług, zapewnienia wygodnego korzystania ze strony oraz w celu monitorowania ruchu zgodnie z Polityką prywatności. Dane są także zbierane i przetwarzane przez narzędzie Google Analytics (więcej).
Możesz zmienić ustawienia cookies w swojej przeglądarce. Ograniczenie stosowania plików cookies w konfiguracji przeglądarki może wpłynąć na niektóre funkcjonalności dostępne na stronie.
Możesz zmienić ustawienia cookies w swojej przeglądarce. Ograniczenie stosowania plików cookies w konfiguracji przeglądarki może wpłynąć na niektóre funkcjonalności dostępne na stronie.