Online first
Bieżący numer
Archiwum
O czasopiśmie
Polityka etyki publikacyjnej
System antyplagiatowy
Instrukcje dla Autorów
Instrukcje dla Recenzentów
Rada Redakcyjna
Komitet Redakcyjny
Recenzenci
Wszyscy recenzenci
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
Kontakt
Bazy indeksacyjne
Klauzula przetwarzania danych osobowych (RODO)
PRACA ORYGINALNA
Wstępna ocena częstości występowania przeciwciał anty-Babesia spp. w grupie osób zawodowo narażonych na pokłucie przez kleszcze w Polsce
1
Instytut Medycyny Wsi, Lublin, Polska
Autor do korespondencji
Anna Kloc
Instytut Medycyny Wsi im. Witolda Chodźki w Lublinie, Jaczewskiego 2, 20-090, Lublin, Polska
Instytut Medycyny Wsi im. Witolda Chodźki w Lublinie, Jaczewskiego 2, 20-090, Lublin, Polska
Med Og Nauk Zdr. 2023;29(1):46-56
SŁOWA KLUCZOWE
DZIEDZINY
STRESZCZENIE
Wprowadzenie i cel pracy:
Pierwotniaki z rodzaju Babesia, w tym Babesia microti, stanowią czynnik etiologiczny ludzkiej babeszjozy. W Polsce głównym wektorem pierwotniaków Babesia spp. są kleszcze z gatunku Ixodes ricinus. Celem badań było wykrycie specyficznych przeciwciał, będących odpowiedzią na zakażenie ludzką babeszjozą, u osób szczególnie narażonych na pokłucie przez kleszcze z uwagi na wykonywany zawód.
Materiał i metody:
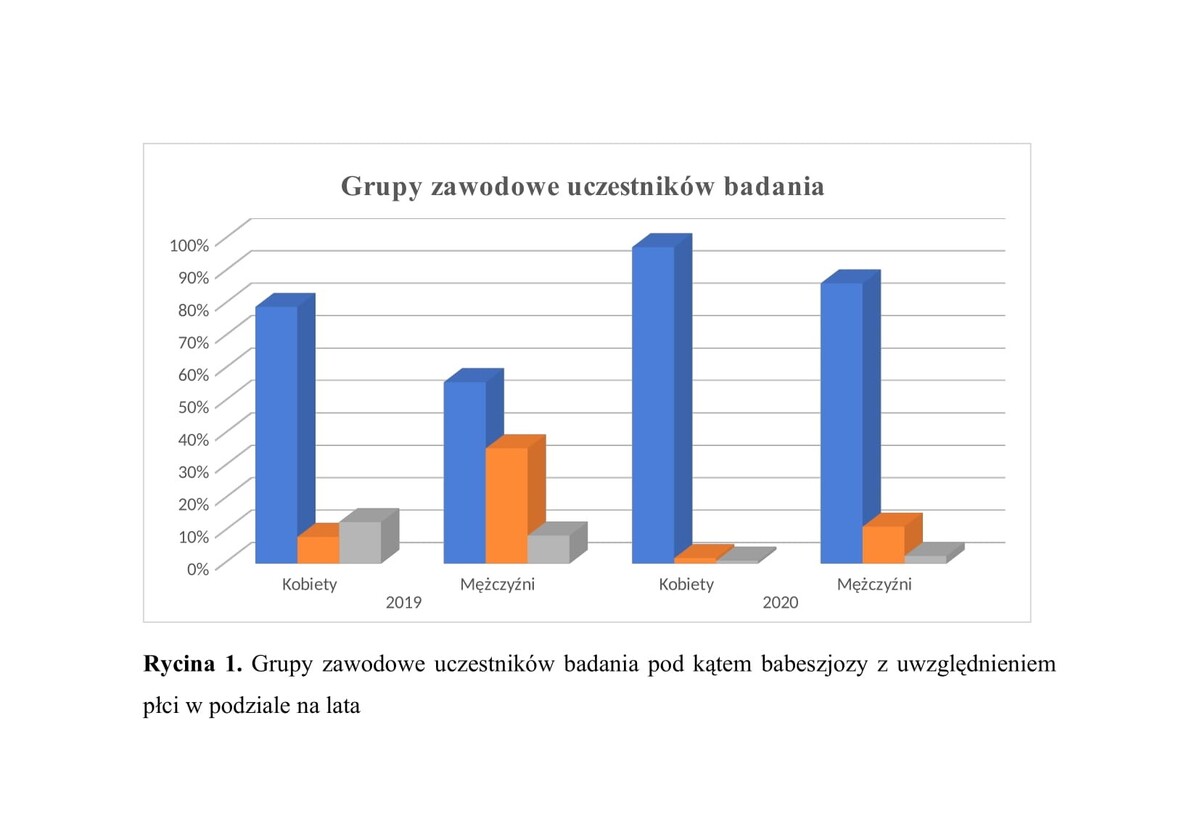
Badaniami objęto grupę 413 osób biorących udział w projekcie w ramach Narodowego Programu Zdrowia. Uczestnicy byli badani w ciągu 2 lat, przy czym w 2019 roku zbadano 203 osoby oraz w 2020 roku – 210 osób. W surowicy krwi pobranej od uczestników badania oznaczono poziom specyficznych przeciwciał w klasie IgG skierowanych przeciw pierwotniakom Babesia za pomocą testu z zakresu immunofluorescencji pośredniej (IFA) z wykorzystaniem komercyjnych zestawów diagnostycznych.
Wyniki:
W grupie 413 uczestników w przeciągu 2 lat odnotowano 3 osoby (0,73% badanych), u których wykryto obecność przeciwciał przeciwko pierwotniakom z rodzaju Babesia na poziomie najniższego miana przeciwciał w klasie IgG, wynoszącego 64. W roku 2019 wykryto 2 przypadki, stanowiące 0,99% badanych, oraz w 2020 roku – jeden przypadek, stanowiący 0,48% badanych.
Wnioski:
W Polsce wyniki badań grup zawodowych szczególnie narażonych na pokłucie przez kleszcze wskazują, że ludzka babeszjoza nie stanowi znaczącego problemu w grupie chorób odkleszczowych. Świadczą o tym wyniki badań serologicznych, gdzie stwierdzono obecność przeciwciał przeciwko pierwotniakom z rodzaju Babesia (wartość miana wynosiła 64) na poziomie 0,73% łącznie w okresie 2 lat. Wskazuje to również na możliwość zarażenia się pierwotniakami z rodzaju Babesia i zachorowania na babeszjozę, co pokazują wyniki innych badań prowadzonych na terenie Polski, gdzie uzyskano znacznie wyższe wyniki prewalencji
Pierwotniaki z rodzaju Babesia, w tym Babesia microti, stanowią czynnik etiologiczny ludzkiej babeszjozy. W Polsce głównym wektorem pierwotniaków Babesia spp. są kleszcze z gatunku Ixodes ricinus. Celem badań było wykrycie specyficznych przeciwciał, będących odpowiedzią na zakażenie ludzką babeszjozą, u osób szczególnie narażonych na pokłucie przez kleszcze z uwagi na wykonywany zawód.
Materiał i metody:
Badaniami objęto grupę 413 osób biorących udział w projekcie w ramach Narodowego Programu Zdrowia. Uczestnicy byli badani w ciągu 2 lat, przy czym w 2019 roku zbadano 203 osoby oraz w 2020 roku – 210 osób. W surowicy krwi pobranej od uczestników badania oznaczono poziom specyficznych przeciwciał w klasie IgG skierowanych przeciw pierwotniakom Babesia za pomocą testu z zakresu immunofluorescencji pośredniej (IFA) z wykorzystaniem komercyjnych zestawów diagnostycznych.
Wyniki:
W grupie 413 uczestników w przeciągu 2 lat odnotowano 3 osoby (0,73% badanych), u których wykryto obecność przeciwciał przeciwko pierwotniakom z rodzaju Babesia na poziomie najniższego miana przeciwciał w klasie IgG, wynoszącego 64. W roku 2019 wykryto 2 przypadki, stanowiące 0,99% badanych, oraz w 2020 roku – jeden przypadek, stanowiący 0,48% badanych.
Wnioski:
W Polsce wyniki badań grup zawodowych szczególnie narażonych na pokłucie przez kleszcze wskazują, że ludzka babeszjoza nie stanowi znaczącego problemu w grupie chorób odkleszczowych. Świadczą o tym wyniki badań serologicznych, gdzie stwierdzono obecność przeciwciał przeciwko pierwotniakom z rodzaju Babesia (wartość miana wynosiła 64) na poziomie 0,73% łącznie w okresie 2 lat. Wskazuje to również na możliwość zarażenia się pierwotniakami z rodzaju Babesia i zachorowania na babeszjozę, co pokazują wyniki innych badań prowadzonych na terenie Polski, gdzie uzyskano znacznie wyższe wyniki prewalencji
Introduction and objective:
Protozoan parasites of the genus Babesia, mainly Babesia microti, are the etiologic factor of human babesiosis, which is one of the emerging diseases. In Poland, the main vector of Babesia spp. are ticks of the species Ixodes ricinus.The aim of the study was to detect specific antibodies to human babesiosis in people who are particularly exposed to tick bites due to their profession.
Material and methods:
The material for the study was a group of 413 participants of the public health project under the National Health Programme. Participants were tested over 2 years – 203 in 2019, and 210 in 2020. The level of specific IgG antibodies against human babesiosis in blood serum of participants was determined by means of an indirect immunofluorescence test (IFA) using commercial diagnostic kits.
Results:
In the group of 413 study participants, 3 cases were found over 2 years (0.73%), in whom the presence of antibodies against babesiosis was detected at the level of the lowest titre of IgG antibodies of 64. In 2019, 2 cases were detected, representing 0.99% of those surveyed, and in 2020, 1 case representing 0.48%.
Conclusions:
In Poland, the results of studies of occupational groups especially exposed to tick bites indicate that babesiosis is not a significant problem in the group of tick-borne diseases. This is evidenced by the results of serological tests, where the presence of antibodies against babesiosis was found at the lowest titre of 64, at a total level of 0.73% over 2 years. This also indicates the potential for infection with protozoa of the genus Babesia and contracting babesiosis, as shown by the results of other studies conducted in Poland, where significantly higher prevalence rates were obtained.
Protozoan parasites of the genus Babesia, mainly Babesia microti, are the etiologic factor of human babesiosis, which is one of the emerging diseases. In Poland, the main vector of Babesia spp. are ticks of the species Ixodes ricinus.The aim of the study was to detect specific antibodies to human babesiosis in people who are particularly exposed to tick bites due to their profession.
Material and methods:
The material for the study was a group of 413 participants of the public health project under the National Health Programme. Participants were tested over 2 years – 203 in 2019, and 210 in 2020. The level of specific IgG antibodies against human babesiosis in blood serum of participants was determined by means of an indirect immunofluorescence test (IFA) using commercial diagnostic kits.
Results:
In the group of 413 study participants, 3 cases were found over 2 years (0.73%), in whom the presence of antibodies against babesiosis was detected at the level of the lowest titre of IgG antibodies of 64. In 2019, 2 cases were detected, representing 0.99% of those surveyed, and in 2020, 1 case representing 0.48%.
Conclusions:
In Poland, the results of studies of occupational groups especially exposed to tick bites indicate that babesiosis is not a significant problem in the group of tick-borne diseases. This is evidenced by the results of serological tests, where the presence of antibodies against babesiosis was found at the lowest titre of 64, at a total level of 0.73% over 2 years. This also indicates the potential for infection with protozoa of the genus Babesia and contracting babesiosis, as shown by the results of other studies conducted in Poland, where significantly higher prevalence rates were obtained.
Kloc A, Galinska EM. Wstępna ocena częstości występowania przeciwciał anty-Babesia spp. w grupie osób zawodowo narażonych na pokłucie
przez kleszcze w Polsce. Med Og Nauk Zdr. 2023; 29(1): 46–56. doi: 10.26444/monz/158846
REFERENCJE (95)
1.
Krause PJ. Human babesiosis. Int J Parasitol. 2019;49(2):165–174. https:// doi.org/10.1016/j.ijpara.2018.11.007.
2.
Rożej-Bielicka W, Stypułkowska-Misiurewicz H, Gołąb E. Human babesiosis. Przegl Epidemiol. 2015; 69(3):489–94,605–8.
3.
Herwaldt BL, Linden JV, Bosserman E, et al. Transfusion-associated babesiosis in the United States: a description of cases. Ann InternMed. 2011;155(8):509–19. https://doi.org/10.7326/0003-4...- 201110180-00362.
4.
Aydin MF, Aktas M, Dumanli N. Molecular identification of Theileria and Babesia in ticks collected from sheep and goats in the Black Sea region of Turkey. Parasitol Res. 2015; 114(1):65–9. https://doi.org/10.1007/ s00436-014-4160-x.
5.
Gray J, Zintl A, Hildebrandt A, et al. Zoonotic babesiosis: overview of the disease and novel aspects of pathogen identity. Ticks Tick Borne Dis. 2010;1(1):3–10. https://doi.org/10.1016/j.ttbd....
6.
Krause PJ, Gewurz BE, Hill D, et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis. 2008;46(3):370–6. https://doi.org/10.1086/525852. PMID: 18181735.
7.
Hildebrandt A, Hunfeld KP. Humane Babesiose – eine seltene, aber po- tenziell gefährliche Zoonose [Human babesiosis – a rare but potentially dangerous zoonosis]. Dtsch Med Wochenschr. 2014;139(18):957–62. German. https://doi.org/10.1055/s-0034....
9.
Katargina O, Geller J, Vasilenko V, et al. Detection and characterization of Babesia species in Ixodes ticks in Estonia. Vector Borne Zoonotic Dis. 2011;11(7):923–8. https://doi.org/10.1089/vbz.20....
10.
Pawełczyk A, Bednarska M, Hamera A, et al. Long-term study of Borrelia and Babesia prevalence and co-infection in Ixodes ricinus and Derma- centor recticulatus ticks removed from humans in Poland, 2016–2019. Parasit Vectors. 2021;14(1):348. https://doi.org/10.1186/s13071....
11.
Lemieux JE, Tran AD, Freimark L, et al. A global map of genetic diversity in Babesia microti reveals strong population structure and identifies variants associated with clinical relapse. Nat Microbiol. 2016;1(7):16079. https://doi.org/10.1038/nmicro....
12.
Silva JC, Cornillot E, McCracken C, et al. Genome-wide diversity and gene expression profiling of Babesia microti isolates identify poly- morphic genes that mediate host-pathogen interactions. Sci Rep. 2016; 6:35284. https://doi.org/10.1038/srep35....
13.
Vannier EG, Diuk-Wasser MA, Ben Mamoun C, et al. Infect Dis Clin North Am. 2015;29(2):357–70. https://doi.org/10.1016/j.idc.....
14.
Jiang JF, Zheng YC, Jiang RR, et al. Epidemiological, clinical, and la- boratory characteristics of 48 cases of „Babesia venatorum” infection in China: a descriptive study. Lancet Infect Dis. 2015;15(2):196–203.https://doi.org/10.1016/S1473-....
15.
Wójcik-Fatla A, Zając V, Sawczyn A, et al. Babesia spp. in questing ticks from eastern Poland: prevalence and species diversity. Parasitol Res. 2015;114(8):3111–6. https://doi.org/10.1007/s00436....
16.
Woolley AE, Montgomery MW, Savage WJ, et al. Post-Babesiosis Warm Autoimmune Hemolytic Anemia. N Engl J Med. 2017;376(10):939–946. https://doi.org/10.1056/NEJMoa.... PMID: 28273010.
17.
Mareedu N, Schotthoefer AM, Tompkins J, et al. Risk Factors for Severe Infection, Hospitalization, and Prolonged Antimicrobial Therapy in Patients with Babesiosis. Am J Trop Med Hyg. 2017;97(4):1218–1225. https://doi.org/10.4269/ajtmh.....
18.
Patel KM, Johnson JE, Reece R, et al. Babesiosis-associated Splenic Rupture: Case Series From a Hyperendemic Region. Clin Infect Dis. 2019;69(7):1212–1217. https://doi.org/10.1093/cid/ci....
19.
Raffalli J, Wormser GP. Persistence of babesiosis for 2 years in a patient on rituximab for rheumatoid arthritis. Diagn Microbiol Infect Dis. 2016;85(2):231–2. https://doi.org/10.1016/j.diag....
20.
Joseph JT, Roy SS, Shams N, et al. Babesiosis in Lower Hudson Val- ley, New York, USA. Emerg Infect Dis. 201;17(5):843–7. https://doi.org/10.3201/eid170....
21.
Vannier E, Krause PJ. Human babesiosis. N Engl J Med. 2012; 366(25):2397–407. https://doi.org/10.1056/NEJMra....
22.
Moritz ED, Winton CS, Tonnetti L, et al. Screening for Babesia microti in the U.S. Blood Supply. N Engl J Med. 2016;375(23):2236–2245. https:// doi.org/10.1056/NEJMoa1600897.
23.
Welc-Falęciak R, Bajer A, Paziewska-Harris A, et al. Diversity of Babesia in Ixodes ricinus ticks in Poland. Adv Med Sci. 2012;57(2):364–9. https:// doi.org/10.2478/v10039-012-0023-9.
24.
Kumar S, Fish D, Krause PJ. Community-acquired and transfusion-transmitted babesiosis are increasing: why and what to do Transfusion. 2018;58(3):617–619. https://doi.org/10.1111/trf.14....
25.
Hilpertshauser H, Deplazes P, Schnyder M, et al. Babesia spp. identified by PCR in ticks collected from domestic and wild ruminants in southern Switzerland. Appl Environ Microbiol. 2006;72(10):6503–7. https://doi. org/10.1128/AEM.00823-06.
26.
Hildebrandt A, Gray JS, Hunfeld KP. Human babesiosis in Europe: what clinicians need to know. Infection. 2013;41(6):1057–72. https://doi.org/10.1007/s15010....
27.
Westblade LF, Simon MS, Mathison BA, et al. Babesia microti: from Mice to Ticks to an Increasing Number of Highly Susceptible Humans. J Clin Microbiol. 2017;55(10):2903–2912. https://doi.org/10.1128/ JCM.00504-17.
28.
Skrabalo Z, Deanovic Z. Piroplasmosis in man; report of a case. Doc Med Geogr Trop. 1957;9:11–16.
29.
Clark IA, Alleva LM, Mills AC, et al. Pathogenesis of malaria and clinically similar conditions. Clin Microbiol Rev. 2004;17(3):509–39, table of contents. https://doi.org/10.1128/CMR.17....
30.
Krause PJ, Daily J, Telford SR, et al. Shared features in the pathobiology of babesiosis and malaria. Trends Parasitol. 2007;23(12):605–10. https:// doi.org/10.1016/j.pt.2007.09.005.
31.
Hatcher JC, Greenberg PD, Antique J, et al. Severe babesiosis in Long Island: review of 34 cases and their complications. Clin Infect Dis. 2001; 32(8):1117–25. https://doi.org/10.1086/319742.
32.
Lobo CA, Singh M, Rodriguez M. Human babesiosis: recent advances and future challenges. Curr Opin Hematol. 2020;27(6):399–405. https:// doi.org/10.1097/MOH.0000000000000606.
33.
Krause PJ, Gewurz BE, Hill D, et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis. 2008;46(3):370–6. https://doi.org/10.1086/525852.
34.
Krause PJ, McKay K, Gadbaw J, et al. Tick-Borne Infection Study Group. Increasing health burden of human babesiosis in endemic sites. Am J Trop Med Hyg. 2003;68(4):431–6.
35.
Wilson M, Glaser KC, Adams-Fish D, et al. Development of droplet digital PCR for the detection of Babesia microti and Babesia duncani. Exp Parasitol. 2015;149:24–31. https://doi.org/10.1016/j.expp....
36.
Grabias B, Clement J, Krause PJ, et al. Superior real-time polymerase chain reaction detection of Babesia microti parasites in whole blood utilizing high-copy BMN antigens as amplification targets. Transfusion. 018;58(8):1924–1932. https://doi.org/10.1111/trf.14....
37.
Levin AE, Williamson PC, Bloch EM, et al. Serologic screening of United States blood donors for Babesia microti using an investigational enzyme immunoassay. Transfusion. 2016;56(7):1866–74. https://doi. org/10.1111/trf.13618.
38.
Kmieciak W, Ciszewski M, Szewczyk EM. Tick-borne diseases in Poland: Prevalence and difficulties in diagnostics. Med Pr. 2016;67(1):73–87. https://doi.org/10.13075/mp.58....
40.
Sanchez E, Vannier E, Wormser GP, et al. Diagnosis, Treatment, and Prevention of Lyme Disease, Human Granulocytic Anaplasmosis, and Babesiosis: A Review. JAMA. 2016;315(16):1767–77. https://doi. org/10.1001/jama.2016.2884.
41.
Senanayake SN, Paparini A, Latimer M, et al. First report of human babesiosis in Australia. Med J Aust. 2012;196(5):350–2. https://doi.org/10.5694/mja11.....
42.
Bullard JM, Ahsanuddin AN, Perry AM, et al. The first case of locally acquired tick-borne Babesia microti infection in Canada. Can J Infect Dis Med Microbiol. 2014;25(6):e87–9. https://doi.org/10.1155/2014/2....
43.
Zhou X, Xia S, Huang JL, et al. Human babesiosis, an emerging tic borne disease in the People›s Republic of China. Parasit Vectors. 2014; 7:509. https://doi.org/10.1186/s13071....
44.
Zhou X, Xia S, Yin SQ, et al. Emergence of babesiosis in China-Myan-mar border areas. Parasit Vectors. 2015;8:390. https://doi.org/10.1186/ s13071-015-0978-z.
45.
Peniche-Lara G, Balmaceda L, Perez-Osorio C, et al. Human Babesiosis, Yucatán State, Mexico, 2015. Emerg Infect Dis. 2018;24(11):2061–2062. https://doi.org/10.3201/eid241....
46.
Zintl A, Mulcahy G, Skerrett HE, et al. Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance. Clin Microbiol Rev. 2003;16(4):622–36. https://doi.org/10.1128/CMR.16....
47.
Hunfeld KP, Hildebrandt A, Gray JS. Babesiosis: recent insights into an ancient disease. Int J Parasitol. 2008;38(11):1219–37. https://doi. org/10.1016/j.ijpara.2008.03.001.
48.
Welc-Falęciak R, Pawełczyk A, Radkowski M, et al. First report of two asymptomatic cases of human infection with Babesia microti (Franca, 1910) in Poland. Ann Agric Environ Med. 2015;22(1):51–4. https://doi. org/10.5604/12321966.1141394.
49.
Welc-Falęciak R, Hildebrandt A, Siński E. Coinfection with Borrelia species and other tick-borne pathogens in humans: two cases from Poland. Ann Agric Environ Med. 2010;17(2):309–13.
50.
Bajer A, Beck A, Beck R, et al. Babesiosis in Southeastern, Central and Northeastern Europe: An Emerging and Re-Emerging Tick-Borne Disease of Humans and Animals. Microorganisms. 2022;10(5):945. https://doi.org/10.3390/microo....
51.
Banović P, Díaz-Sánchez AA, Simin V, et al. Clinical aspects and detection of emerging rickettsial pathogens: A “One Health” approach study in Serbia, 2020. Front Microbiol. 2021;12:797399. https://doi. org/10.3389/fmicb.2021.797399.
52.
Berger S. Infectious Diseases of Luxembourg: 2021 Edition. Los Angeles, CA, USA: GIDEON Informatics, Inc.; 2021.
53.
Bondarenko AV, Torianyk II, Pokhil SI, et al. Seroprevalence of babesiosis in immunocompetent and immunocompromised individuals. Pol Merkur Lekarski. 2021;49(291):193–197.
54.
Dedkov VG, Simonova EG, Beshlebova OV, et al. The burden of tick borne diseases in the Altai region of Russia. Ticks Tick Borne Dis. 2017;8(5):787–794. https://doi.org/10.1016/j.ttbd....
55.
Topolovec J, Puntarić D, Antolović-Pozgain A, et al. Serologically detected “new” tickborne zoonoses in Eastern Croatia. Croat Med J. 2003;44(5):626–9.
56.
Herwaldt BL, Caccio S, Gherlinzoni F, et al. Molecular characterization of a non-Babesia divergens organism causing zoonotic babesiosis in Europe. Emerg Infect Dis. 2003;9:942–948. https://doi.org/10.3201/eid090....
57.
Blum S, Gattringer R, Haschke E, et al. The case: Hemolysis and acute renal failure. Babesiosis. Kidney Int. 2011;80:681–683. https://doi. org/10.1038/ki.2011.184.
58.
Ramharter M, Walochnik J, Lagler H, et al. Clinical and molecular characterization of a near fatal case of human babesiosis in Austria. J Travel Med. 2010;17:416–418. https://doi.org/10.1111/j.1708....
59.
Nohýnková E, Kubek J, Mĕst’ánková O, et al. A case of Babesia microti imported into the Czech Republic from the USA. Cas Lek Cesk. 2003; 142(6):377–81.
60.
Strizova Z, Havlova K, Patek O, et al. The first human case of babesiosis mimicking Reiter’s syndrome. Folia Parasitol. 2020; 67:1–5. https://doi.org/10.14411/fp.20....
61.
Hildebrandt A, Hunfeld KP, Baier M, et al. First confirmed autochthonous case of human Babesia microti infection in Europe. Eur J Clin Microbiol Infect Dis. 2007;26:595–601. https://doi.org/10.1007/ s10096-007-0333-1.
62.
Berens-Riha N, Zechmeister M, Hirzmann J, et al. Babesiose bei einem splenektomierten Reisenden aus den USA–Nach Deutschland importierte Infektion durch Zecken. Flugmed. Tropenmed. Reisemed. 2012;19:113–115.
63.
Häselbarth K, Tenter AM, Brade V, et al. First case of human babesiosis in Germany – Clinical presentation and molecular characterisation of the pathogen. Int J Med Microbiol. 2007;297(3):197–204. https://doi. org/10.1016/j.ijmm.2007.01.002.
64.
Duh D, Slovák M, Saksida A, et al. Molecular detection of Babesia canis in Dermacentor reticulatus ticks collected in Slovakia. Biologia. 2006;61:231–233. https://doi.org/10.2478/s11756....
65.
Mørch K, Holmaas G, Frolander PS, et al. Severe human Babesia diver- gens infection in Norway. Int J Infect Dis. 2015;33:37–38. https://doi. org/10.1016/j.ijid.2014.12.034.
66.
Mysterud A, Stigum VM, Seland IV, et al. Tick abundance, pathogen prevalence, and disease incidence in two contrasting regions at the northern distribution range of Europe. Parasit Vectors. 2018;11(1):309. https://doi.org/10.1186/s13071....
67.
Uhnoo I, Cars O, Christensson D, et al. First documented case of human babesiosis in Sweden. Scand J Infect Dis. 1992;24(4):541–7. https://doi. org/10.3109/00365549209052642.
68.
Wilhelmsson P, Lövmar M, Krogfelt KA, et al. Clinical/serological outcome in humans bitten by Babesia species positive Ixodes ricinus ticks in Sweden and on the Åland Islands. Ticks Tick Borne Dis. 2020; 11:101455. https://doi.org/10.101 /j.ttbdis.2020.101455.
69.
Svensson J, Hunfeld KP, Persson KEM. High seroprevalence of Babesia antibodies among Borrelia burgdorferi-infected humans in Sweden. Ticks Tick Borne Dis. 2019;10(1):186–190. https://doi.org/10.1016/j. ttbdis.2018.10.007.
70.
Humiczewska M, Kuźna-Grygiel W. A case of imported human babesiosis in Poland. Wiad Parazytol. 1997;43:227–229.
71.
Przybylińska A, Bielicki D, Kuźna-Grygiel W. The case of babesiosis in a patient with ulcerative colitis treated with immunosuppressive drugs. Gastroenterol Pol. 2004;11:607–609.
72.
Welc-Falęciak R, Hildebrandt A, Siński E. Co-infection with Borrelia species and other tick-borne pathogens in humans: Two cases from Poland. Ann Agric Environ Med. 2010;17:309–313.
73.
Jabłońska J, Żarnowska-Prymek H, Stańczak J, et al. Symptomatic coinfection with Babesia microti and Borrelia burgdorferi in patient after international exposure; a challenging case in Poland. Ann Agric Envi- ron Med. 2016;23:387–389. https://doi.org/10.5604/123219....
74.
Moniuszko-Malinowska A, Guziejko K, Czarnowska A, et al. Assessment of anti-HSV antibodies in patients with facial palsy in the course of neuroborreliosis. Int J Clin Pract. 2021;75:e13749. https://doi. org/10.1111/ijcp.13749.
75.
Moniuszko-Malinowska A, Dunaj J, Andersson MO, et al. Assessment of Anaplasma phagocytophilum presence in early Lyme borreliosis manifested by erythema migrans skin lesions. Travel Med Infect Dis. 2020;36:101648. https://doi.org/10.1016/j.tmai....
76.
Moniuszko-Malinowska A, Dunaj J, Andersson MO, et al. Anaplasmosis in Poland—Analysis of 120 patients. Ticks Tick Borne Dis. 2021;12:101763. https://doi.org/10.1016/j.ttbd....
77.
Krawczuk K, Czupryna P, Pancewicz S, et al. Comparison of tick-borne encephalitis between children and adults—Analysis of 669 patients. J Neurovirol. 2020;26:565–571. https://doi.org/10.1007/s13365....
78.
Pancewicz S, Moniuszko A, Bieniarz E, et al. Anti-Babesia microti antibodies in foresters highly exposed to tick bites in Poland. Scand J Infect Dis. 2011;197–201. https://doi.org/10.3109/003655....
79.
Moniuszko-Malinowska A, Swiecicka I, Dunaj J, et al. Infection with Babesia microti in humans with non-specific symptoms in North East Poland. Infect Dis. 2016;48:537–543. https://doi.org/10.3109/237442... 5.2016.1164339.
80.
Moniuszko A, Dunaj J, Swięcicka I, et al. Co-infections with Borrelia species, Anaplasma phagocytophilum and Babesia spp. in patients with tick-borne encephalitis. Eur J Clin Microbiol Infect Dis. 2014; 33:1835–1841. https://doi.org/10.1007/s10096....
81.
Dunaj J, Moniuszko-Malinowska A, Swiecicka I, et al. Tick-borne infections and co-infections in patients with non-specific symptoms in Poland. Adv Med Sci. 2018;63:167–172. https://doi.org/10.1016/j. advms.2017.09.004.
82.
Pawełczyk A, Bednarska M, Kowalska JD, et al. Seroprevalence of six pathogens transmitted by the Ixodes ricinus ticks in asymptomatic individuals with HIV infection and in blood donors. Sci Rep. 2019; 9:2117. https://doi.org/10.1038/s41598....
83.
Adaszek Ł, Winiarczyk S, Łukaszewska J. Babesiose bei einer Katze. Kleintierpraxis. 2010;55:624–634.
85.
Kretschmer M. (Vetlab sp. z o.o., Veterinary Diagnostic Laboratory, Wrocław, Poland). Personal communication. 2022.
86.
Staniec M, Buczek K, Milczak A, et al. Epidemiological studies towards cattle babesiosis-tick-borne disease. Scient Messeng LNU Vet Med Biotech. 2016;18:228–239. https://doi.org/10.15421/nvlve....
87.
Staniec M, Adaszek Ł, Buczek K, et al. Molecular identification of Babesia spp. isolated from Polish cattle with asymptomatic protozoa infections. Pol J Vet Sci. 2018;21:287–291. https://doi.org/10.24425/11905....
88.
Wójcik-Fatla A, Bartosik K, Buczek A, et al. Babesia microti in adult Dermacentor reticulatus ticks from eastern Poland. Vector Borne Zoonotic Dis. 2012;12(10):841–3. https://doi.org/10.1089/vbz.20....
89.
Grochowska A, Dunaj J, Pancewicz S, et al. Detection of Borrelia burgdorferi s.l., Anaplasma phagocytophilum and Babesia spp. in Dermacentor reticulatus ticks found within the city of Białystok, Poland-first data. Exp Appl Acarol. 2021;85(1):63–73. https://doi.org/10.1007/ s10493-021-00655-x.
90.
Asman M, Witecka J, Solarz K, et al. Occurrence of Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Babesia microti in Ixodes ricinus ticks collected from selected areas of Opolskie Province in south-west Poland. Ann Agric Environ Med. 2019;26(4):544–547. https://doi.org/.26444/aaem/11....
91.
Kletsova EA, Spitzer ED, Fries BC, et al. Babesiosis in Long Island: review of 62 cases focusing on treatment with azithromycin and atovaquone. Ann Clin Microbiol Antimicrob. 2017;16(1):26. https://doi. org/10.1186/s12941-017-0198-9.
92.
Saifee NH, Krause PJ, Wu Y. Apheresis for babesiosis: Therapeutic parasite reduction or removal of harmful toxins or both? J Clin Apher. 2016;31(5):454–8. https://doi.org/10.1002/jca.21....
93.
Finch C, Al-Damluji MS, Krause PJ, et al. Integrated assessment of behavioral and environmental risk factors for Lyme disease infection on Block Island, Rhode Island. PLoS One. 2014;9(1):e84758. https://doi. org/10.1371/journal.pone.0084758.
94.
Schetters TP, Moubri K, Cooke BM. Comparison of Babesia rossi and Babesia canis isolates with emphasis on effects of vaccination with soluble parasite antigens: a review. J S Afr Vet Assoc. 2009;80(2):75–8. https://doi.org/10.4102/jsava.....
95.
Teodorowski O, Kalinowski M, Skrzypczak M, et al. Babesia gibsoni infection in dogs in Poland. Pol J Vet Sci. 2020;23:469–471. https://doi. org/10.24425/pjvs.2020.134694.
Udostępnij
ARTYKUŁ POWIĄZANY
Przetwarzamy dane osobowe zbierane podczas odwiedzania serwisu. Realizacja funkcji pozyskiwania informacji o użytkownikach i ich zachowaniu odbywa się poprzez dobrowolnie wprowadzone w formularzach informacje oraz zapisywanie w urządzeniach końcowych plików cookies (tzw. ciasteczka). Dane, w tym pliki cookies, wykorzystywane są w celu realizacji usług, zapewnienia wygodnego korzystania ze strony oraz w celu monitorowania ruchu zgodnie z Polityką prywatności. Dane są także zbierane i przetwarzane przez narzędzie Google Analytics (więcej).
Możesz zmienić ustawienia cookies w swojej przeglądarce. Ograniczenie stosowania plików cookies w konfiguracji przeglądarki może wpłynąć na niektóre funkcjonalności dostępne na stronie.
Możesz zmienić ustawienia cookies w swojej przeglądarce. Ograniczenie stosowania plików cookies w konfiguracji przeglądarki może wpłynąć na niektóre funkcjonalności dostępne na stronie.