Online first
Bieżący numer
Archiwum
O czasopiśmie
Polityka etyki publikacyjnej
System antyplagiatowy
Instrukcje dla Autorów
Instrukcje dla Recenzentów
Rada Redakcyjna
Komitet Redakcyjny
Recenzenci
Wszyscy recenzenci
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
Kontakt
Bazy indeksacyjne
Klauzula przetwarzania danych osobowych (RODO)
PRACA PRZEGLĄDOWA
Tirzepatyd – innowacyjna metoda leczenia cukrzycy i otyłości
1
Kliniczny Oddział Toksykologiczno-Kardiologiczny i Chorób Wewnętrznych, Wojewódzki Szpital Specjalistyczny im. Stefana Kardynała Wyszyńskiego SPZOZ w Lublinie, Polska
2
Wydział Lekarski, Uniwersytet Medyczny w Lublinie, Polska
3
Oddział Geriatrii, Samodzielny Publiczny Zakład Opieki Zdrowotnej w Łęcznej, Polska
Autor do korespondencji
Krystian Żuk
Kliniczny Oddział Toksykologiczno-Kardiologiczny i Chorób Wewnętrznych, Wojewódzki Szpital Specjalistyczny im. Stefana Kardynała Wyszyńskiego SPZOZ w Lublinie, Al. Kraśnicka 100, 20-718 Lublin, Polska
Kliniczny Oddział Toksykologiczno-Kardiologiczny i Chorób Wewnętrznych, Wojewódzki Szpital Specjalistyczny im. Stefana Kardynała Wyszyńskiego SPZOZ w Lublinie, Al. Kraśnicka 100, 20-718 Lublin, Polska
Med Og Nauk Zdr. 2025;31(2):108-113
SŁOWA KLUCZOWE
DZIEDZINY
STRESZCZENIE
Wprowadzenie i cel:
Otyłość dotyka milionów ludzi na świecie i jest podłożem wielu poważnych schorzeń, takich jak cukrzyca typu 2 czy choroby układu krążenia. Niniejszy artykuł miał na celu analizę badań serii SURPASS i SURMOUNT dotyczących skuteczności i bezpieczeństwa tirzepatydu jako innowacyjnego leku stosowanego w terapii cukrzycy oraz otyłości.
Metody przeglądu:
Przeglądu literatury dokonano przy użyciu baz danych PubMed i Google Scholar. Przeanalizowano publikacje z lat 2021–2025, a jako kryteria wyszukiwania zastosowano następujące słowa kluczowe: „tirzepatyd”, „otyłość”, „cukrzyca”, „inkretyny”.
Opis stanu wiedzy:
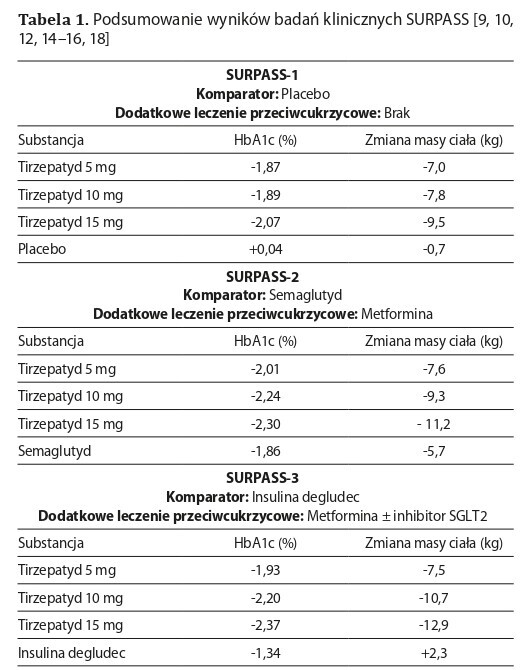
Hormony inkretynowe, takie jak GLP-1 i GIP, odgrywają kluczową rolę w regulacji poziomu cukru we krwi, apetytu i masy ciała. Tirzepatyd, nowatorski lek, łączy działanie obu inkretyn i wykazuje wysoką skuteczność w leczeniu cukrzycy typu 2 oraz otyłości. Badania kliniczne serii SURPASS i SURMOUNT potwierdziły, że tirzepatyd redukuje masę ciała i poprawia kontrolę glikemii w porównaniu z placebo, insuliną oraz innymi agonistami GLP-1. Lek ten jest dobrze tolerowany, a jego dodatkowe korzyści obejmują poprawę ciśnienia krwi oraz funkcji nerek i zmniejszenie otłuszczenia wątroby.
Podsumowanie:
Otyłość i cukrzyca typu 2 to globalne wyzwania zdrowotne, wymagające skutecznych terapii. Tirzepatyd wykazał w badaniach klinicznych wyższą efektywność niż metody wpisujące się w dotychczasowe standardy leczenia. Lek ten jest bezpieczny (charakteryzuje się korzystnym profilem bezpieczeństwa) i zapewnia poprawę parametrów metabolicznych. Konieczne są jednak dalsze badania na większych populacjach, by zwiększyć wiarygodność uzyskanych wyników. Odkrycie to może zrewolucjonizować w przyszłości leczenie chorób metabolicznych.
Otyłość dotyka milionów ludzi na świecie i jest podłożem wielu poważnych schorzeń, takich jak cukrzyca typu 2 czy choroby układu krążenia. Niniejszy artykuł miał na celu analizę badań serii SURPASS i SURMOUNT dotyczących skuteczności i bezpieczeństwa tirzepatydu jako innowacyjnego leku stosowanego w terapii cukrzycy oraz otyłości.
Metody przeglądu:
Przeglądu literatury dokonano przy użyciu baz danych PubMed i Google Scholar. Przeanalizowano publikacje z lat 2021–2025, a jako kryteria wyszukiwania zastosowano następujące słowa kluczowe: „tirzepatyd”, „otyłość”, „cukrzyca”, „inkretyny”.
Opis stanu wiedzy:
Hormony inkretynowe, takie jak GLP-1 i GIP, odgrywają kluczową rolę w regulacji poziomu cukru we krwi, apetytu i masy ciała. Tirzepatyd, nowatorski lek, łączy działanie obu inkretyn i wykazuje wysoką skuteczność w leczeniu cukrzycy typu 2 oraz otyłości. Badania kliniczne serii SURPASS i SURMOUNT potwierdziły, że tirzepatyd redukuje masę ciała i poprawia kontrolę glikemii w porównaniu z placebo, insuliną oraz innymi agonistami GLP-1. Lek ten jest dobrze tolerowany, a jego dodatkowe korzyści obejmują poprawę ciśnienia krwi oraz funkcji nerek i zmniejszenie otłuszczenia wątroby.
Podsumowanie:
Otyłość i cukrzyca typu 2 to globalne wyzwania zdrowotne, wymagające skutecznych terapii. Tirzepatyd wykazał w badaniach klinicznych wyższą efektywność niż metody wpisujące się w dotychczasowe standardy leczenia. Lek ten jest bezpieczny (charakteryzuje się korzystnym profilem bezpieczeństwa) i zapewnia poprawę parametrów metabolicznych. Konieczne są jednak dalsze badania na większych populacjach, by zwiększyć wiarygodność uzyskanych wyników. Odkrycie to może zrewolucjonizować w przyszłości leczenie chorób metabolicznych.
Introduction and objective:
Obesity affects millions of people worldwide and underlies many serious conditions, such as type 2 diabetes and cardiovascular diseases. This article aims to analyze SURPASS and SURMOUNT series of studies on the efficacy and safety of tirzepatide as an innovative drug used in the treatment of diabetes and obesity.
Review methods:
A literature review was conducted using the PubMed and Google Scholar databases. Publications from 2021–2025 were analyzed, using the following keywords as search criteria: ‘tirzepatide’, ‘obesity’, ‘diabetes’, and ‘incretins’.
Abbreviated description of the state of knowledge:
Brief description of the state of knowledge. Incretin hormones, such as GLP-1 and GIP, play a key role in regulating blood sugar levels, appetite, and body weight. Tirzepatide, an innovative drug, combines the action of both incretins and demonstrates high efficacy in treating type 2 diabetes and obesity. Clinical trials of the SURPASS and SURMOUNT series have confirmed that tirzepatide reduces body weight and improves glycaemic control compared to placebo, insulin, and other GLP-1 agonists. This drug is well-tolerated, with additional benefits including improved blood pressure, kidney function, and reduced liver fat.
Summary:
Obesity and type 2 diabetes are global health challenges requiring effective therapies. Tirzepatide has demonstrated greater efficacy in clinical trials than the existing treatment standards. The drug is characterized by a favourable safety profile and improvements in metabolic parameters. However; further research on larger populations is necessary to increase the reliability of results. This discovery could revolutionize the treatment of metabolic diseases in the future.
Obesity affects millions of people worldwide and underlies many serious conditions, such as type 2 diabetes and cardiovascular diseases. This article aims to analyze SURPASS and SURMOUNT series of studies on the efficacy and safety of tirzepatide as an innovative drug used in the treatment of diabetes and obesity.
Review methods:
A literature review was conducted using the PubMed and Google Scholar databases. Publications from 2021–2025 were analyzed, using the following keywords as search criteria: ‘tirzepatide’, ‘obesity’, ‘diabetes’, and ‘incretins’.
Abbreviated description of the state of knowledge:
Brief description of the state of knowledge. Incretin hormones, such as GLP-1 and GIP, play a key role in regulating blood sugar levels, appetite, and body weight. Tirzepatide, an innovative drug, combines the action of both incretins and demonstrates high efficacy in treating type 2 diabetes and obesity. Clinical trials of the SURPASS and SURMOUNT series have confirmed that tirzepatide reduces body weight and improves glycaemic control compared to placebo, insulin, and other GLP-1 agonists. This drug is well-tolerated, with additional benefits including improved blood pressure, kidney function, and reduced liver fat.
Summary:
Obesity and type 2 diabetes are global health challenges requiring effective therapies. Tirzepatide has demonstrated greater efficacy in clinical trials than the existing treatment standards. The drug is characterized by a favourable safety profile and improvements in metabolic parameters. However; further research on larger populations is necessary to increase the reliability of results. This discovery could revolutionize the treatment of metabolic diseases in the future.
Żuk K, Góral A, Duszyńska, Dolepski K, Czachajda, Kot A. Tirzepatyd – innowacyjna metoda leczenia cukrzycy i otyłości. Med Og Nauk Zdr.
2025; 31(2): 108–113. doi: 10.26444/monz/205172
REFERENCJE (25)
1.
Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022;387(3):205–216. https://doi.org/10.1056/NEJMOA....
2.
Corrao S, Pollicino C, Maggio D, et al. Tirzepatide against obesity and insulin-resistance: pathophysiological aspects and clinical evidence. Front Endocrinol (Lausanne). 2024;15. https://doi.org/10.3389/FENDO.....
3.
De Block C, Bailey C, Wysham C, et al. Tirzepatide for the treatment of adults with type 2 diabetes: An endocrine perspective. Diabetes Obes Metab. 2022;25(1):3. https://doi.org/10.1111/DOM.14....
4.
Bosch C, Carriazo S, Soler MJ, et al. Tirzepatide and prevention of chronic kidney disease. Clin Kidney J. 2022;16(5):797. https://doi.org/10.1093/CKJ/SF....
5.
Fisman EZ, Tenenbaum A. The dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist tirzepatide: a novel cardiometabolic therapeutic prospect. Cardiovasc Diabetol. 2021;20(1):1–5. https://doi.org/10.1186/S12933...
6.
Chen SY, Kong XQ, Zhang KF, et al. DPP4 as a Potential Candidate in Cardiovascular Disease. J Inflamm Res. 2022;15:5457–5469. https://doi.org/10.2147/JIR.S3....
7.
Nauck MA, D‘Alessio DA. Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovasc Diabetol. 2022;21(1):169. https://doi.org/10.1186/S12933....
8.
Forzano I, Varzideh F, Avvisato R, et al. Tirzepatide: A Systematic Update. Int J Mol Sci. 2022;23(23). https://doi.org/10.3390/IJMS23....
9.
Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet (London, England). 2021;398(10295):143–155. https://doi.org/10.1016/S0140-....
10.
Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N Engl J Med. 2021;385(6):503–515. https://doi.org/10.1056/NEJMOA....
11.
Frias JP, De Block C, Brown K, et al. Tirzepatide Improved Markers of Islet Cell Function and Insulin Sensitivity in People With T2D (SURPASS-2). J Clin Endocrinol Metab. 2024;109(7):1745–1753. https://doi.org/10.1210/CLINEM....
12.
Ludvik B, Giorgino F, Jódar E, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet (London, England). 2021;398(10300):583–598. https://doi.org/10.1016/S0140-....
13.
Gastaldelli A, Cusi K, Fernández Landó L, et al. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. lancet Diabetes Endocrinol. 2022;10(6):393–406. https://doi.org/10.1016/S2213-....
14.
Del Prato S, Kahn SE, Pavo I, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet (London, England). 2021;398(10313):1811–1824. https://doi.org/10.1016/S0140-....
15.
Dahl D, Onishi Y, Norwood P, et al. Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial. JAMA. 2022;327(6):534. https://doi.org/10.1001/JAMA.2....
16.
Rosenstock J, Frías JP, Rodbard HW, et al. Tirzepatide vs Insulin Lispro Added to Basal Insulin in Type 2 Diabetes: The SURPASS-6 Randomized Clinical Trial. JAMA. 2023;330(17):1631–1640. https://doi.org/10.1001/JAMA.2....
17.
Nicholls SJ, Bhatt DL, Buse JB, et al. Comparison of tirzepatide and dulaglutide on major adverse cardiovascular events in participants with type 2 diabetes and atherosclerotic cardiovascular disease: SURPASS-CVOT design and baseline characteristics. Am Heart J. 2024;267:1–11. https://doi.org/10.1016/J.AHJ.....
18.
Andraos J, Muhar H, Smith SR. Beyond glycemia: Comparing tirzepatide to GLP-1 analogues. Rev Endocr Metab Disord. 2023;24(6):1089. https://doi.org/10.1007/S11154....
19.
le Roux CW, Zhang S, Aronne LJ, et al. Tirzepatide for the treatment of obesity: Rationale and design of the SURMOUNT clinical development program. Obesity (Silver Spring). 2023;31(1):96–110. https://doi.org/10.1002/OBY.23....
20.
Krumholz HM, De Lemos JA, Sattar N, et al. Tirzepatide and blood pressure reduction: stratified analyses of the SURMOUNT-1 randomised controlled trial. Heart. 2024;110(19). https://doi.org/10.1136/HEARTJ....
21.
Garvey WT, Frias JP, Jastreboff AM, et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet (London, England). 2023;402(10402):613–626. https://doi.org/10.1016/S0140-....
22.
Wadden TA, Chao AM, Machineni S, et al. Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: the SURMOUNT-3 phase 3 trial. Nat Med. 2023;29(11):2909–2918. https://doi.org/10.1038/S41591....
23.
Aronne LJ, Sattar N, Horn DB, et al. Continued Treatment With Tirzepatide for Maintenance of Weight Reduction in Adults With Obesity: The SURMOUNT-4 Randomized Clinical Trial. JAMA. 2024;331(1):38–48. https://doi.org/10.1001/JAMA.2....
24.
Malhotra A, Grunstein RR, Fietze I, et al. Tirzepatide for the Treatment of Obstructive Sleep Apnea and Obesity. N Engl J Med. 2024;391(13). https://doi.org/10.1056/NEJMOA....
25.
Rubino DM, Pedersen SD, Connery L, et al. Gastrointestinal tolerability and weight reduction associated with tirzepatide in adults with obesity or overweight with and without type 2 diabetes in the SURMOUNT-1 to -4 trials. Diabetes Obes Metab. Published online 2025. https://doi.org/10.1111DOM.161....
Udostępnij
ARTYKUŁ POWIĄZANY
Przetwarzamy dane osobowe zbierane podczas odwiedzania serwisu. Realizacja funkcji pozyskiwania informacji o użytkownikach i ich zachowaniu odbywa się poprzez dobrowolnie wprowadzone w formularzach informacje oraz zapisywanie w urządzeniach końcowych plików cookies (tzw. ciasteczka). Dane, w tym pliki cookies, wykorzystywane są w celu realizacji usług, zapewnienia wygodnego korzystania ze strony oraz w celu monitorowania ruchu zgodnie z Polityką prywatności. Dane są także zbierane i przetwarzane przez narzędzie Google Analytics (więcej).
Możesz zmienić ustawienia cookies w swojej przeglądarce. Ograniczenie stosowania plików cookies w konfiguracji przeglądarki może wpłynąć na niektóre funkcjonalności dostępne na stronie.
Możesz zmienić ustawienia cookies w swojej przeglądarce. Ograniczenie stosowania plików cookies w konfiguracji przeglądarki może wpłynąć na niektóre funkcjonalności dostępne na stronie.