Online first
Bieżący numer
Archiwum
O czasopiśmie
Polityka etyki publikacyjnej
System antyplagiatowy
Instrukcje dla Autorów
Instrukcje dla Recenzentów
Rada Redakcyjna
Komitet Redakcyjny
Recenzenci
Wszyscy recenzenci
2024
2023
2022
2021
2020
2019
2018
2017
2016
Kontakt
Bazy indeksacyjne
Klauzula przetwarzania danych osobowych (RODO)
PRACA PRZEGLĄDOWA
Witamina D3 – fundamentalny komponent zdrowia człowieka oraz potencjalny suplement w zapobieganiu i terapii COVID-19
1
Zakład Biologii Molekularnej i Badań Translacyjnych, Instytut Medycyny Wsi im. Witolda Chodźki w Lublinie, Polska
Autor do korespondencji
Krzysztof Bogumił Sawicki
Zakład Biologii Molekularnej i Badań Translacyjnych, Instytut Medycyny Wsi im. Witolda Chodźki w Lublinie, Jaczewskiego 2, 20-090, Lublin, Polska
Zakład Biologii Molekularnej i Badań Translacyjnych, Instytut Medycyny Wsi im. Witolda Chodźki w Lublinie, Jaczewskiego 2, 20-090, Lublin, Polska
Med Og Nauk Zdr. 2021;27(3):227-234
SŁOWA KLUCZOWE
DZIEDZINY
STRESZCZENIE
Wprowadzenie i cel:
Witamina D3 jest jednym z najbardziej istotnych związków endogennych syntetyzowanych w organizmie człowieka. Pierwotnie jej niedobór powiązano z występowaniem krzywicy u dzieci. Jednakże liczne badania przeprowadzone w ciągu ostatnich dziesięcioleci wskazały, że witamina D3 ma dużo większe znaczenie, niż wcześniej przypuszczano. Celem niniejszej pracy była analiza dostępnej literatury naukowej dotyczącej witaminy D3 i jej wpływu na zdrowie człowieka.
Metody przeglądu:
W celu przeszukiwania bazy danych Pubmed użyto następujących kombinacji słów kluczowych: [„vitamin D”] + [„synthesis”, „metabolism”, „receptor”, „epidemiology”, „deficiency”, „SARS-CoV-2”]. Po zastosowaniu kry-teriów wykluczających do przeglądu wybrano 67 publikacji
Opis stanu wiedzy:
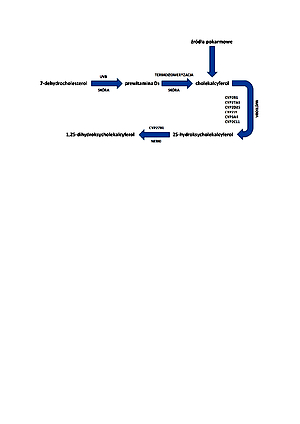
Około 90% witaminy D3 jest wytwarzane w skórze w rezultacie ekspozycji na promieniowanie UVB. Po-została część jest dostarczana z żywnością lub suplementami diety. Postuluje się, że niedobór witaminy D3 jest obecnie jednym z najbardziej palących problemów zdrowotnych. Dane literaturowe wskazują, że niedobór witaminy D3 poważnie zaburza homeostazę organizmu i zwiększa ryzyko powstawania niektórych nowotworów, chorób sercowo-naczyniowych, a także cukrzycy typu 2. W związku z wybuchem pandemii koronawirusa SARS-CoV-2 w 2019 roku rozpoczęto badania kliniczne dotyczące wpływu witaminy D3 na podatność na infekcję i przebieg choroby. Stwierdzono jej korzystny wpływ w przeciwdziałaniu zakażeniom i łagodzeniu skutków COVID-19.
Podsumowanie:
Wraz z postępem medycyny zrozumiano, jak ważna dla prawidłowego funkcjonowania organizmu jest witamina D3 i jakie konsekwencje zdrowotne powoduje jej niedobór. Dlatego z punktu widzenia zdrowia publicznego istotne jest powszechne uświadomienie społeczeństwa o znaczeniu tej witaminy dla zdrowia i zaletach jej regularnego przyjmowania.
Witamina D3 jest jednym z najbardziej istotnych związków endogennych syntetyzowanych w organizmie człowieka. Pierwotnie jej niedobór powiązano z występowaniem krzywicy u dzieci. Jednakże liczne badania przeprowadzone w ciągu ostatnich dziesięcioleci wskazały, że witamina D3 ma dużo większe znaczenie, niż wcześniej przypuszczano. Celem niniejszej pracy była analiza dostępnej literatury naukowej dotyczącej witaminy D3 i jej wpływu na zdrowie człowieka.
Metody przeglądu:
W celu przeszukiwania bazy danych Pubmed użyto następujących kombinacji słów kluczowych: [„vitamin D”] + [„synthesis”, „metabolism”, „receptor”, „epidemiology”, „deficiency”, „SARS-CoV-2”]. Po zastosowaniu kry-teriów wykluczających do przeglądu wybrano 67 publikacji
Opis stanu wiedzy:
Około 90% witaminy D3 jest wytwarzane w skórze w rezultacie ekspozycji na promieniowanie UVB. Po-została część jest dostarczana z żywnością lub suplementami diety. Postuluje się, że niedobór witaminy D3 jest obecnie jednym z najbardziej palących problemów zdrowotnych. Dane literaturowe wskazują, że niedobór witaminy D3 poważnie zaburza homeostazę organizmu i zwiększa ryzyko powstawania niektórych nowotworów, chorób sercowo-naczyniowych, a także cukrzycy typu 2. W związku z wybuchem pandemii koronawirusa SARS-CoV-2 w 2019 roku rozpoczęto badania kliniczne dotyczące wpływu witaminy D3 na podatność na infekcję i przebieg choroby. Stwierdzono jej korzystny wpływ w przeciwdziałaniu zakażeniom i łagodzeniu skutków COVID-19.
Podsumowanie:
Wraz z postępem medycyny zrozumiano, jak ważna dla prawidłowego funkcjonowania organizmu jest witamina D3 i jakie konsekwencje zdrowotne powoduje jej niedobór. Dlatego z punktu widzenia zdrowia publicznego istotne jest powszechne uświadomienie społeczeństwa o znaczeniu tej witaminy dla zdrowia i zaletach jej regularnego przyjmowania.
Introduction and objective:
Vitamin D3 is one of the most essential endogenous compounds synthesized in the human body. Originally, its deficiency was associated with the occu-rrence of rickets in children. However, a number of studies carried out over the last decades indicated that vitamin D3 is much more important than previously thought. The aim of this study was to analyze the available scientific literature on vitamin D3 and its effects on human health.
Review methods:
The following combinations of key words were used to search the Pubmed database: [vitamin D] + [synthesis, metabolism, receptor, epidemiology, deficiency, SARS-CoV-2]. After applying exclusion criteria, 67 articles were selected for review.
Abbreviated description of the state of knowledge:
About 90% of vitamin D3 is produced in the skin as a result of exposure to UVB radiation. The remaining portion is supplied from food or dietary supplements. It is postulated that vitamin D3 deficiency is one of the most pressing health problems today. Literature data indicate that vitamin D3 deficiency severely disrupts the body’s homeostasis and increases the risk of certain cancers, cardiovascular diseases, and type 2 diabetes. Since the 2019 outbreak of the SARS-CoV-2 coronavirus pan-demic, clinical studies have been initiated on the effects of vitamin D3 on susceptibility to infection and disease course. It has been found to have beneficial effects in preventing infection and mitigating the effects of COVID-19.
Summary:
With the progress of medicine it has been under-stood how important vitamin D3 is for the proper functioning of the body and what health consequences its deficiency entails. Therefore, from the point of view of public health it is important to make the general public aware of the im-portance of this vitamin for health and the advantages of its regular intake.
Vitamin D3 is one of the most essential endogenous compounds synthesized in the human body. Originally, its deficiency was associated with the occu-rrence of rickets in children. However, a number of studies carried out over the last decades indicated that vitamin D3 is much more important than previously thought. The aim of this study was to analyze the available scientific literature on vitamin D3 and its effects on human health.
Review methods:
The following combinations of key words were used to search the Pubmed database: [vitamin D] + [synthesis, metabolism, receptor, epidemiology, deficiency, SARS-CoV-2]. After applying exclusion criteria, 67 articles were selected for review.
Abbreviated description of the state of knowledge:
About 90% of vitamin D3 is produced in the skin as a result of exposure to UVB radiation. The remaining portion is supplied from food or dietary supplements. It is postulated that vitamin D3 deficiency is one of the most pressing health problems today. Literature data indicate that vitamin D3 deficiency severely disrupts the body’s homeostasis and increases the risk of certain cancers, cardiovascular diseases, and type 2 diabetes. Since the 2019 outbreak of the SARS-CoV-2 coronavirus pan-demic, clinical studies have been initiated on the effects of vitamin D3 on susceptibility to infection and disease course. It has been found to have beneficial effects in preventing infection and mitigating the effects of COVID-19.
Summary:
With the progress of medicine it has been under-stood how important vitamin D3 is for the proper functioning of the body and what health consequences its deficiency entails. Therefore, from the point of view of public health it is important to make the general public aware of the im-portance of this vitamin for health and the advantages of its regular intake.
Sawicki K, Skawiński W. Witamina D3 – fundamentalny komponent zdrowia człowieka oraz potencjalny suplement w zapobieganiu i terapii COVID-19. Med Og Nauk Zdr. Doi: 10.26444/monz/140406
REFERENCJE (68)
1.
Machado Cda S, Venancio VP, Aissa AF, Hernandes LC, de Mello MB, Del Lama JE, Marzocchi-Machado CM, Bianchi ML, Antunes LM. Vitamin D3 deficiency increases DNA damage and the oxidative burst of neutrophils in a hypertensive rat model. Mutat Res Genet Toxicol Environ Mutagen. 2016 Mar; 798–799: 19–26. doi: 10.1016/j.mrgentox.2016.01.005.
2.
Mahmoodani F, Perera CO, Fedrizzi B, Abernethy G, Chen H. De-gradation studies of cholecalciferol (vitamin D3) using HPLC-DAD, UHPLC-MS/MS and chemical derivatization. Food Chem. 2017 Mar 15; 219: 373–381. doi: 10.1016/j.foodchem.2016.09.146.
3.
Yu C, Fedoric B, Anderson PH, Lopez AF, Grimbaldeston MA. Vitamin D(3) signalling to mast cells: A new regulatory axis. Int J Biochem Cell Biol. 2011 Jan; 43(1): 41–6. doi: 10.1016/ j.biocel.2010.10.011.
4.
Orlova T, Moan J, Lagunova Z, Aksnes L, Terenetskaya I, Juzeniene A. Increase in serum 25-hydroxyvitamin-D3 in humans after sunbed exposures compared to previtamin D3 synthesis in vitro. J Photochem Photobiol B. 2013 May 5; 122: 32–6. doi: 10.1016/ j.jphotobiol.2013.03.006.
5.
Chiellini G, Rapposelli S, Zhu J, Massarelli I, Saraceno M, Bianucci.AM, Plum LA, Clagett-Dame M, DeLuca HF. Synthesis and biological activities of vitamin D-like inhibitors of CYP24 hydroxylase. Steroids. 2012 Feb; 77(3): 212–23. doi: 10.1016/j.steroids.2011.11.007.
6.
Jorde R, Grimnes G. Serum cholecalciferol may be a better marker of vitamin D status than 25-hydroxyvitamin D. Med Hypotheses. 2018 Feb; 111: 61–65. doi: 10.1016/ j.mehy. 2017.12.017.
7.
Li W, Chen J, Janjetovic Z, Kim TK, Sweatman T, Lu Y, Zjawiony J, Tuckey RC, Miller D, Slominski A. Chemical synthesis of 20S-hy-droxyvitamin D3, which shows antiproliferative activity. Steroids. 2010 Dec; 75(12): 926–35. doi: 10.1016/ j.steroids.2010.05.021.
8.
Yasuda K, Endo M, Ikushiro S, Kamakura M, Ohta M, Sakaki T. UV-de-pendent production of 25-hydroxyvitamin D2 in the recombinant yeast cells expressing human CYP2R1. Biochem Biophys Res Commun. 2013 May 3; 434(2): 311–5. doi: 10.1016/j.bbrc.2013.02.124.
9.
Bikle DD, Patzek S, Wang Y. Physiologic and pathophysiologic roles of extra renal CYP27b1: Case report and review. Bone Rep. 2018 Feb 26; 8: 255–267. doi: 10.1016/j.bonr.2018.02.004.
10.
Garach-Jehoshua O, Ravid A, Liberman UA, Koren R. 1,25-Dihy-droxyvitamin D3 increases the growth-promoting activity of autocrine epidermal growth factor receptor ligands in keratinocytes. Endocrino-logy. 1999 Feb; 140(2): 713–21. doi: 10.1210/endo.140.2.6520.
11.
Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014 Mar 20; 21(3): 319–29. doi: 10.1016/j.chembiol.2013.12.016.
12.
Slominski AT, Brożyna AA, Zmijewski MA, Jóźwicki W, Jetten AM, Mason RS, Tuckey RC, Elmets CA. Vitamin D signaling and mela-noma: role of vitamin D and its receptors in melanoma progression and management. Lab Invest. 2017 Jun; 97(6): 706–724. doi: 10.1038/labinvest.2017.3.
13.
Hünten S, Hermeking H. p53 directly activates cystatin D/CST5 to mediate mesenchymal-epithelial transition: a possible link to tumor suppression by vitamin D3. Oncotarget. 2015 Jun 30; 6(18): 15842–56. doi: 10.18632/oncotarget.4683.
14.
Pinheiro MM, Fabbri A, Infante M. Cytokine storm modulation in COVID-19: a proposed role for vitamin D and DPP-4 inhibitor com-bination therapy (VIDPP-4i). Immunotherapy. 2021 Apr 28: 10.2217/imt-2020-0349. doi: 10.2217/imt-2020-0349.
15.
Hart PH, Gorman S. Exposure to UV Wavelengths in Sunlight Suppres-ses Immunity. To What Extent is UV-induced Vitamin D3 the Mediator Responsible? Clin Biochem Rev. 2013 Feb; 34(1): 3–13.
16.
Kechichian E, Ezzedine K. Vitamin D and the Skin: An Update for Dermatologists. Am J Clin Dermatol. 2018 Apr; 19(2): 223–235. doi: 10.1007/s40257-017-0323-8.
17.
Sikoglu EM, Navarro AA, Starr D, Dvir Y, Nwosu BU, Czerniak SM, Rogan RC, Castro MC, Edden RA, Frazier JA, Moore CM. Vitamin D3 Supplemental Treatment for Mania in Youth with Bipolar Spectrum Disorders. J Child Adolesc Psychopharmacol. 2015 Jun; 25(5): 415–24. doi: 10.1089/cap.2014.0110.
18.
Enciso PL, Wang L, Kawahara Y, Sakamoto S, Shimada S, Takeichi Y, Takayanagi R, Nomura M. Dietary vitamin D3 improves postprandial hyperglycemia in aged mice. Biochem Biophys Res Commun. 2015 May 22; 461(1): 165–71. doi: 10.1016/j.bbrc.2015.04.008.
19.
Myszka M, Klinger M. Immunomodulacyjne działanie witaminy D [The immunomodulatory role of Vitamin D]. Postepy Hig Med Dosw (Online). 2014; 68: 865–78. Polish. doi: 10.5604/17322693.1110168.
20.
Xue Y, Ying L, Horst RL, Watson G, Goltzman D. Androgens Attenuate Vitamin D Production Induced by UVB Irradiation of the Skin of Male Mice by an Enzymatic Mechanism. J Invest Dermatol. 2015 Dec; 135(12): 3125–3132. doi: 10.1038/jid.2015.297.
21.
Shin MH, Lee Y, Kim MK, Lee DH, Chung JH. UV increases skin--derived 1α,25-dihydroxyvitamin D3 production, leading to MMP-1 expression by altering the balance of vitamin D and cholesterol synthesis from 7-dehydrocholesterol. J Steroid Biochem Mol Biol. 2019 Dec; 195: 105449. doi: 10.1016/j.jsbmb.2019.105449.
22.
Bolerazska B, Rabajdova M, Spakova I, Marekova M. Current knowledge on the active form of Vitamin D synthesized in the skin and its effects on malignant melanoma. Neoplasma. 2017; 64(1): 1–12. doi: 10.4149/neo_2017_101.
23.
Piotrowska A, Wierzbicka J, Ślebioda T, Woźniak M, Tuckey RC, Slo-minski AT, Żmijewski MA. Vitamin D derivatives enhance cytotoxic effects of H2O2 or cisplatin on human keratinocytes. Steroids. 2016 Jun; 110: 49–61. doi: 10.1016/j.steroids.2016.04.002.
24.
Khammissa RAG, Fourie J, Motswaledi MH, Ballyram R, Lemmer J, Feller L. The Biological Activities of Vitamin D and Its Receptor in Relation to Calcium and Bone Homeostasis, Cancer, Immune and Cardiovascular Systems, Skin Biology, and Oral Health. Biomed Res Int. 2018 May 22; 2018: 9276380. doi: 10.1155/2018/9276380.
25.
Reichrath J, Saternus R, Vogt T. Endocrine actions of vitamin D in skin: Relevance for photocarcinogenesis of non-melanoma skin cancer, and beyond. Mol Cell Endocrinol. 2017 Sep 15; 453: 96–102. doi: 10.1016/j.mce.2017.05.001.
26.
Kosmowska-Miśków A. The role of vitamin D3 in inflammatory bowel diseases. Adv Clin Exp Med. 2014 Jul-Aug; 23(4): 497–504. doi: 10.17219/acem/37208.
27.
Lehmann B, Meurer M. Vitamin D metabolism. Dermatol Ther. 2010 Jan-Feb; 23(1): 2–12. doi: 10.1111/j.1529-8019.2009.01286.x.
28.
Di Rosa M, Malaguarnera M, Zanghì A, Passaniti A, Malaguarnera L. Vitamin D3 insufficiency and colorectal cancer. Crit Rev Oncol Hematol. 2013 Dec; 88(3): 594–612. doi: 10.1016/j.critrevonc.2013.07.016.
29.
Barry EL, Rees JR, Peacock JL, Mott LA, Amos CI, Bostick RM, Figue-iredo JC, Ahnen DJ, Bresalier RS, Burke CA, Baron JA. Genetic variants in CYP2R1, CYP24A1, and VDR modify the efficacy of vitamin D3 supplementation for increasing serum 25-hydroxyvitamin D levels in a randomized controlled trial. J Clin Endocrinol Metab. 2014 Oct; 99(10): E2133–7. doi: 10.1210/jc.2014-1389.
30.
Boontanrart M, Hall SD, Spanier JA, Hayes CE, Olson JK. Vitamin D3 alters microglia immune activation by an IL-10 dependent SOCS3 mechanism. J Neuroimmunol. 2016 Mar 15; 292: 126–36. doi: 10.1016/j.jneuroim.2016.01.015.
31.
Chen PZ, Li M, Duan XH, Jia JY, Li JQ, Chu RA, Yu C, Han JH, Wang H. Pharmacokinetics and effects of demographic factors on blood 25(OH)D3 levels after a single orally administered high dose of vitamin D3. Acta Pharmacol Sin. 2016 Nov; 37(11): 1509–1515. doi: 10.1038/aps.2016.82.
32.
Ralph AP, Lucas RM, Norval M. Vitamin D and solar ultraviolet ra-diation in the risk and treatment of tuberculosis. Lancet Infect Dis. 2013 Jan; 13(1): 77–88. doi: 10.1016/S1473-3099(12)70275-X. Erratum in: Lancet Infect Dis. 2013 Feb; 13(2): 106. Ralph, Anna R [corrected to Ralph, Anna P]. Erratum in: Lancet Infect Dis. 2013 Mar; 13(3): 192.
33.
Deb S, Pandey M, Adomat H, Guns ES. Cytochrome P450 3A-media-ted microsomal biotransformation of 1α,25-dihydroxyvitamin D3 in mouse and human liver: drug-related induction and inhibition of catabolism. Drug Metab Dispos. 2012 May; 40(5): 907–18. doi: 10.1124/dmd.111.041681.
34.
Norlin M, Lundqvist J, Ellfolk M, Hellström Pigg M, Gustafsson J, Wikvall K. Drug-Mediated Gene Regulation of Vitamin D3 Metabolism in Primary Human Dermal Fibroblasts. Basic Clin Pharmacol Toxicol. 2017 Jan; 120(1): 59–63. doi: 10.1111/bcpt.12641.
35.
Wu W, Fan H, Jiang Y, Liao L, Li L, Zhao J, Zhang H, Shrestha C, Xie Z. Regulation of 25-hydroxyvitamin D-1-hydroxylase and 24-hydroxylase in keratinocytes by PTH and FGF23. Exp Dermatol. 2018 Nov; 27(11): 1201–1209. doi: 10.1111/exd.13760.
36.
Shang M, Sun J. Vitamin D/VDR, Probiotics, and Gastrointestinal Diseases. Curr Med Chem. 2017; 24(9): 876–887. doi: 10.2174/0929867323666161202150008.
37.
Uberti F, Bardelli C, Morsanuto V, Ghirlanda S, Molinari C. Role of vitamin D3 combined to alginates in preventing acid and oxidative injury in cultured gastric epithelial cells. BMC Gastroenterol. 2016 Oct 7; 16(1): 127. doi: 10.1186/s12876-016-0543-z.
38.
Saksa N, Neme A, Ryynänen J, Uusitupa M, de Mello VD, Voutila-inen S, Nurmi T, Virtanen JK, Tuomainen TP, Carlberg C. Dissec-ting high from low responders in a vitamin D3 intervention study. J Steroid Biochem Mol Biol. 2015 Apr; 148: 275–82. doi: 10.1016/j.jsbmb.2014.11.012.
39.
Acar S, Demir K, Shi Y. Genetic Causes of Rickets. J Clin Res Pediatr Endocrinol. 2017 Dec 30; 9(Suppl 2): 88–105. doi: 10.4274/jcrpe.2017.S008.
40.
Lucas J, Badia JL, Lucas E, Remon A. Cinacalcet treatment experience in hereditary vitamin D resistant rickets. J Pediatr Endocrinol Metab. 2020 Feb 25; 33(2): 313–318. doi: 10.1515/jpem-2019-0258.
41.
Bakke D, Sun J. Ancient Nuclear Receptor VDR With New Functions: Microbiome and Inflammation. Inflamm Bowel Dis. 2018 May 18; 24(6): 1149–1154. doi: 10.1093/ibd/izy092.
42.
Lee SM, Meyer MB, Benkusky NA, O'Brien CA, Pike JW. The impact of VDR expression and regulation in vivo. J Steroid Biochem Mol Biol. 2018 Mar; 177: 36–45. doi: 10.1016/j.jsbmb.2017.06.002.
43.
Guo C, Rosoha E, Lowry MB, Borregaard N, Gombart AF. Curcumin induces human cathelicidin antimicrobial peptide gene expression through a vitamin D receptor-independent pathway. J Nutr Biochem. 2013 May;24(5): 754–9. doi: 10.1016/j.jnutbio.2012.04.002.
44.
Trochoutsou AI, Kloukina V, Samitas K, Xanthou G. Vitamin-D in the Immune System: Genomic and Non-Genomic Actions. Mini Rev Med Chem. 2015; 15(11): 953–63. doi: 10.2174/1389557515666150519110830.
45.
Feister U, Laschewski G, Grewe RD. UV index forecasts and measure-ments of health-effective radiation. J Photochem Photobiol B. 2011 Jan 10; 102(1): 55–68. doi: 10.1016/j.jphotobiol.2010.09.005.
46.
Nair-Shalliker V, Clements M, Fenech M, Armstrong BK. Personal sun exposure and serum 25-hydroxy vitamin D concentrations. Pho-tochem Photobiol. 2013 Jan-Feb; 89(1): 208–14. doi: 10.1111/j.1751--1097.2012.01201.x.
47.
Buczkowski K, Chlabicz S, Dytfeld J, Horst-Sikorska W, Jaroszyński A, Kardas P, Marcinkowska M, Siebert J, Tałałaj M. Wytyczne dla lekarzy rodzinnych dotyczące suplementacji witaminy D. Forum Medycyny Rodzinnej 2013; 7(2): 55–58.
48.
Rusińska A, Płudowski P, Walczak M, Borszewska-Kornacka MK, Bossowski A, Chlebna-Sokół D, et al. Vitamin D Supplementation Guidelines for General Population and Groups at Risk of Vitamin D De-ficiency in Poland-Recommendations of the Polish Society of Pediatric Endocrinology and Diabetes and the Expert Panel With Participation of National Specialist Consultants and Representatives of Scientific Societies-2018 Update. Front Endocrinol (Lausanne). 2018 May 31; 9: 246. doi: 10.3389/fendo.2018.00246.
49.
Wacker M, Holick MF. Vitamin D – effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013 Jan 10; 5(1): 111–48. doi: 10.3390/nu5010111.
50.
Napiórkowska L, Franek E. Rola oznaczania witaminy D w praktyce klinicznej. Choroby Serca i Naczyń 2009; 6(4): 203–210.
51.
Lehmann U, Riedel A, Hirche F, Brandsch C, Girndt M, Ulrich C, Seibert E, Henning C, Glomb MA, Dierkes J, Stangl GI. Vitamin D3 supplementation: Response and predictors of vitamin D3 metabolites – A randomized controlled trial. Clin Nutr. 2016 Apr; 35(2): 351–358. doi: 10.1016/j.clnu.2015.04.021.
52.
Alsaqr A, Rasoully M, Musteata FM. Investigating transdermal delivery of vitamin D3. AAPS PharmSciTech. 2015 Aug; 16(4): 963–72. doi: 10.1208/s12249-015-0291-3.
53.
Schuster I. Cytochromes P450 are essential players in the vitamin D signaling system. Biochim Biophys Acta. 2011 Jan; 1814(1): 186–99. doi: 10.1016/j.bbapap.2010.06.022.
54.
de Medeiros Cavalcante IG, Silva AS, Costa MJ, Persuhn DC, Issa CT, de Luna Freire TL, da Conceição Rodrigues Gonçalves M. Effect of vitamin D3 supplementation and influence of BsmI polymorphism of the VDR gene of the inflammatory profile and oxidative stress in elderly women with vitamin D insufficiency: Vitamin D3 megadose reduces inflammatory markers. Exp Gerontol. 2015 Jun; 66: 10–6. doi: 10.1016/j.exger.2015.03.011.
55.
Oliveira Filho RS, Oliveira DA, Martinho VA, Antoneli CB, Marcussi LA, Ferreira CE. Serum level of vitamin D3 in cutaneous melanoma. Einstein (Sao Paulo). 2014 Oct-Dec; 12(4): 473–6. doi: 10.1590/S1679--45082014AO3090.
56.
Marino R, Misra M. Extra-Skeletal Effects of Vitamin D. Nutrients. 2019 Jun 27; 11(7): 1460. doi: 10.3390/nu11071460.
57.
Svensson D, Nebel D, Nilsson BO. Vitamin D3 modulates the innate immune response through regulation of the hCAP-18/LL-37 gene ex-pression and cytokine production. Inflamm Res. 2016 Jan; 65(1): 25–32. doi: 10.1007/s00011-015-0884-z.
58.
Farias AS, Spagnol GS, Bordeaux-Rego P, Oliveira CO, Fontana AG, de Paula RF, et al. Vitamin D3 induces IDO+ tolerogenic DCs and enhances Treg, reducing the severity of EAE. CNS Neurosci Ther. 2013 Apr; 19(4): 269–77. doi: 10.1111/cns.12071.
59.
Lucas RM, Byrne SN, Correale J, Ilschner S, Hart PH. Ultraviolet ra-diation, vitamin D and multiple sclerosis. Neurodegener Dis Manag. 2015 Oct; 5(5): 413–24. doi: 10.2217/nmt.15.33.
60.
Wang R, DeGruttola V, Lei Q, Mayer KH, Redline S, Hazra A, Mora S, Willett WC, Ganmaa D, Manson JE. The vitamin D for COVID-19.
61.
(VIVID) trial: A pragmatic cluster-randomized design. Contemp Clin Trials. 2021 Jan; 100: 106176. doi: 10.1016/j.cct.2020.106176.
62.
Tan CW, Ho LP, Kalimuddin S, Cherng BPZ, Teh YE, Thien SY, et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in ol-der patients with coronavirus (COVID-19). Nutrition. 2020 Nov-Dec; 79–80: 111017. doi: 10.1016/j.nut.2020.111017.
63.
Ling SF, Broad E, Murphy R, Pappachan JM, Pardesi-Newton S, Kong MF, Jude EB. High-Dose Cholecalciferol Booster Therapy is Associated with a Reduced Risk of Mortality in Patients with COVID-19: A Cross--Sectional Multi-Centre Observational Study. Nutrients. 2020 Dec 11; 12(12): 3799. doi: 10.3390/nu12123799.
64.
Annweiler C, Beaudenon M, Gautier J, Simon R, Dubée V, Gonsard J, Parot-Schinkel E; COVIT-TRIAL study group. COvid-19 and high-dose Vitamin D supplementation TRIAL in high-risk older patients (COVIT--TRIAL): study protocol for a randomized controlled trial. Trials. 2020 Dec 28; 21(1): 1031. doi: 10.1186/s13063-020-04928-5.
65.
D'Avolio A, Avataneo V, Manca A, Cusato J, De Nicolò A, Lucchini R, Keller F, Cantù M. 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2. Nutrients. 2020 May 9; 12(5): 1359. doi: 10.3390/nu12051359.
66.
Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020 Jul; 32(7): 1195–1198. doi: 10.1007/s40520-020-01570-8.
67.
Sulli A, Gotelli E, Casabella A, Paolino S, Pizzorni C, Alessandri E, Grosso M, Ferone D, Smith V, Cutolo M. Vitamin D and Lung Outco-mes in Elderly COVID-19 Patients. Nutrients. 2021 Feb 24; 13(3): 717. doi: 10.3390/nu13030717.
68.
Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcalá Díaz JF, López Miranda J, Bouillon R, Quesada Gomez JM. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among pa-tients hospitalized for COVID-19: A pilot randomized clinical stu-dy. J Steroid Biochem Mol Biol. 2020 Oct; 203: 105751. doi: 10.1016/j.jsbmb.2020.105751.
Udostępnij
ARTYKUŁ POWIĄZANY
Przetwarzamy dane osobowe zbierane podczas odwiedzania serwisu. Realizacja funkcji pozyskiwania informacji o użytkownikach i ich zachowaniu odbywa się poprzez dobrowolnie wprowadzone w formularzach informacje oraz zapisywanie w urządzeniach końcowych plików cookies (tzw. ciasteczka). Dane, w tym pliki cookies, wykorzystywane są w celu realizacji usług, zapewnienia wygodnego korzystania ze strony oraz w celu monitorowania ruchu zgodnie z Polityką prywatności. Dane są także zbierane i przetwarzane przez narzędzie Google Analytics (więcej).
Możesz zmienić ustawienia cookies w swojej przeglądarce. Ograniczenie stosowania plików cookies w konfiguracji przeglądarki może wpłynąć na niektóre funkcjonalności dostępne na stronie.
Możesz zmienić ustawienia cookies w swojej przeglądarce. Ograniczenie stosowania plików cookies w konfiguracji przeglądarki może wpłynąć na niektóre funkcjonalności dostępne na stronie.