REVIEW PAPER
Health-promoting potential and microbial composition of fermented drink tepache
1
Pomorski Uniwersytet Medyczny w Szczecinie, Polska
Corresponding author
Karolina Patrycja Jakubczyk
Pomorski Uniwersytet Medyczny w Szczecinie, Broniewskiego, 46, 71-460, SZCZECIN, Polska
Pomorski Uniwersytet Medyczny w Szczecinie, Broniewskiego, 46, 71-460, SZCZECIN, Polska
Med Og Nauk Zdr. 2021;27(3):272-276
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
Fermentation is a well-known and widely practised method of food processing, increasing both the nutritional and sensoric properties of food products. Thanks to the processes initiated by microorganisms, these products are enriched with bioactive peptides and biogenic amines, creating functional food that supports human health. Additionally, plant-based fermented beverages can provide an easily accessible alternative to fermented milk products for people who are lactose intolerant or allergic to milk proteins. The aim of this article was to review studies on the fermented drink tepache in order to determine its pro-health potential and the possibility of using it in the prevention of selected disorders and diseases
State of knowledge:
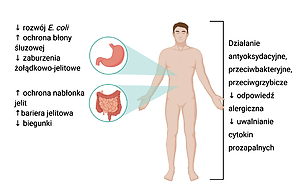
Tepache is a traditional Mexican fermented drink made from pineapple. It is valued for its unique taste and aroma, and because it is easy and quick to make. Strains of bacteria, such as Lactobacillus pentosus, L. paracasei, L. plantarum and L. lactis and yeast from the genus Saccharomyces were isolated from tepache. The properties of microbes found in tepache include support for the host›s normal microflora, modulation of the immune system and regulation of the digestive system. In addition, thanks to its antibiotic and antifungal properties, tepache is a food safe for humans with potential health-promoting effects.
Summary:
Due to the insufficient number of reports, there is a need for extended research documenting the detailed microbiological composition and the impact of tepache on the functioning of the human body
Fermentation is a well-known and widely practised method of food processing, increasing both the nutritional and sensoric properties of food products. Thanks to the processes initiated by microorganisms, these products are enriched with bioactive peptides and biogenic amines, creating functional food that supports human health. Additionally, plant-based fermented beverages can provide an easily accessible alternative to fermented milk products for people who are lactose intolerant or allergic to milk proteins. The aim of this article was to review studies on the fermented drink tepache in order to determine its pro-health potential and the possibility of using it in the prevention of selected disorders and diseases
State of knowledge:
Tepache is a traditional Mexican fermented drink made from pineapple. It is valued for its unique taste and aroma, and because it is easy and quick to make. Strains of bacteria, such as Lactobacillus pentosus, L. paracasei, L. plantarum and L. lactis and yeast from the genus Saccharomyces were isolated from tepache. The properties of microbes found in tepache include support for the host›s normal microflora, modulation of the immune system and regulation of the digestive system. In addition, thanks to its antibiotic and antifungal properties, tepache is a food safe for humans with potential health-promoting effects.
Summary:
Due to the insufficient number of reports, there is a need for extended research documenting the detailed microbiological composition and the impact of tepache on the functioning of the human body
REFERENCES (43)
1.
Lavefve L, Marasini D, Carbonero F. Microbial Ecology of Fermented Vegetables and Non-Alcoholic Drinks and Current Knowledge on Their Impact on Human Health. Adv Food Nutr Res. 2019; 87: 147–85.
2.
Zaręba D, Ziarno M. Alternatywne probiotyczne napoje warzywne i owocowe. Bromat Chem Toksykol. 2011; XLIV: 160–8.
3.
Nguyen BT, Bujna E, Fekete N, et al. Probiotic Beverage From Pineapple Juice Fermented With Lactobacillus and Bifidobacterium Strains. Front Nutr. 2019; 6. doi: 10.3389/fnut.2019.00054.
4.
Gille D, Schmid A, Walther B, et al. Fermented Food and Non-Commu-nicable Chronic Diseases: A Review. Nutrients. 2018; 10. doi: 10.3390/nu10040448.
5.
Selhub EM, Logan AC, Bested AC. Fermented foods, microbiota, and mental health: ancient practice meets nutritional psychiatry. J Physiol Anthropol. 2014; 33: 2.
6.
Baschali A, Tsakalidou E, Kyriacou A, et al. Traditional low-alcoholic and non-alcoholic fermented beverages consumed in European countr-ies: a neglected food group. Nutr Res Rev. 2017; 30: 1–24.
7.
Chen JM, Al KF, Craven LJ, et al. Nutritional, Microbial, and Allergenic Changes during the Fermentation of Cashew ‘Cheese’ Product Using a Quinoa-Based Rejuvelac Starter Culture. Nutrients. 2020; 12. doi: 10.3390/nu12030648.
8.
Corona-González RI, Ramos-Ibarra JR, Gutiérrez-González P, et al. The Use of Response Surface Methodology to Evaluate the Fermen-tation Conditions in the Production of Tepache. Rev Mex Ing Quím. 2013; 12: 19–28.
9.
de la Fuente-Salcido NM, Castañeda-Ramírez JC, García-Almendárez BE et al. Isolation and characterization of bacteriocinogenic lactic bac-teria from M-Tuba and Tepache, two traditional fermented beverages in México. Food Sci Nutr. 2015; 3: 434–42.
10.
Şanlier N, Gökcen BB, Sezgin AC. Health benefits of fermented foods. Crit Rev Food Sci Nutr. 2019; 59: 506–27.
11.
Escobar-Ramírez MC, Jaimez-Ordaz J, Escorza-Iglesias VA, et al. Lac-tobacillus pentosus ABHEAU-05: An in vitro digestion resistant lactic acid bacterium isolated from a traditional fermented Mexican beverage. Rev Argent Microbiol. 2020. doi: 10.1016/j.ram.2019.10.005.
12.
Guatemala-Morales G, González P, Corona R, et al. The use of response surface methodology to evaluate the fermentation conditions in the production of tepache. Rev Mex Ing Quím. 2013; 12: 19–28.
13.
Barrios-Roblero C, Rosas-Quijano R, Salvador-Figueroa M, et al. An-tifungal lactic acid bacteria isolated from fermented beverages with activity against Colletotrichum gloeosporioides. Food Biosci. 2019; 29: 47–54.
14.
Romero-Luna HE, Hernández-Sánchez H, Dávila-Ortiz G. Traditional fermented beverages from Mexico as a potential probiotic source. Ann Microbiol. 2017; 67: 577–86.
15.
Alvarado C, García A, Martin SE, et al. Food-associated lactic acid bacteria with antimicrobial potential from traditional Mexican foods. Rev Latinoam Microbiol. 2006; 48: 260–8.
16.
Bendali F, Kerdouche K, Hamma-Faradji S, et al. In vitro and in vivo cholesterol lowering ability of Lactobacillus pentosus KF923750. Benef Microbes. 2017; 8: 271–80.
17.
Chen Y-H, Wu C-S, Chao Y-H, et al. Lactobacillus pentosus GMNL-77 inhibits skin lesions in imiquimod-induced psoriasis-like mice. J Food Drug Anal. 2017; 25: 559–66.
18.
Behera SS, Ray RC, Zdolec N. Lactobacillus plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. BioMed Res Int. 2018; 2018: 9361614.
19.
Wołkowicz T, Januszkiewicz A, Szych J. [Gut microbiome and its dys-biosis as an important factor influencing the human health condition]. Med Dosw Mikrobiol. 2014; 66: 223–35.
20.
Wang J, Ji H, Wang S, et al. Probiotic Lactobacillus plantarum Promo-tes Intestinal Barrier Function by Strengthening the Epithelium and Modulating Gut Microbiota. Front Microbiol. 2018; 9: 1953.
21.
Cebeci A, Gürakan C. Properties of potential probiotic Lactobacillus plantarum strains. Food Microbiol. 2003; 20: 511–8.
22.
Wołkowicz A, Hozyasz KK. Rola szczepu Lactobacillus plantarum 299v w zapobieganiu i leczeniu zaburzeń układu pokarmowego. Pediatr Pol. 2013; 88: 347–52.
23.
Caridi A. Selection of Escherichia coli-inhibiting strains of Lactobacillus paracasei subsp. paracasei. J Ind Microbiol Biotechnol. 2002; 29: 303–8.
24.
Chiang S-S, Pan T-M. Beneficial effects of Lactobacillus paracasei subsp. paracasei NTU 101 and its fermented products. Appl Microbiol Biotechnol. 2012; 93: 903–16.
25.
Tsai Y-T, Cheng P-C, Liao J-W, et al. Effect of the administration of Lactobacillus paracasei subsp. paracasei NTU 101 on Peyer’s patch--mediated mucosal immunity. Int Immunopharmacol. 2010; 10: 791–8.
26.
Tsai Y-T, Cheng P-C, Fan C-K, et al. Time-dependent persistence of enhanced immune response by a potential probiotic strain Lactoba-cillus paracasei subsp. paracasei NTU 101. Int J Food Microbiol. 2008; 128: 219–25.
27.
Romero-Luna HE, Peredo-Lovillo A, Hernández-Mendoza A, et al. Probiotic Potential of Lactobacillus paracasei CT12 Isolated from Water Kefir Grains (Tibicos). Curr Microbiol. 2020; 77: 2584–92.
28.
Yerlikaya O. Probiotic potential and biochemical and technological properties of Lactococcus lactis ssp. lactis strains isolated from raw milk and kefir grains. J Dairy Sci. 2019; 102: 124–34.
29.
Simčič S, Berlec A, Stopinšek S, et al. Engineered and wild-type L. lactis promote anti-inflammatory cytokine signalling in inflammatory bowel disease patient’s mucosa. World J Microbiol Biotechnol. 2019; 35: 45.
30.
Kumariya R, Garsa AK, Rajput YS, et al. Bacteriocins: Classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microb Pathog. 2019; 128: 171–7.
31.
Ołdak A, Zielińska D. Bakteriocyny bakterii fermentacji mlekowej jako alternatywa antybiotyków. Postępy Hig Med Dośw. 2017; 71. doi: 10.5604/01.3001.0010.3817.
32.
De Vuyst L. Nisin production variability between natural Lactococcus lactis subsp. Lactis strains. Biotechnol Lett. 1994; 16: 287–92.
34.
Cintas LM, Casaus P, Fernández MF, et al. Comparative antimicrobial activity of enterocin L50, pediocin PA-1, nisin A and lactocin S against spoilage and foodborne pathogenic bacteria. Food Microbiol 1998; 15: 289–98.
35.
Schillinger U, Geisen R, Holzapfel WH. Potential of antagonistic mic-roorganisms and bacteriocins for the biological preservation of foods. Trends Food Sci Technol. 1996; 7: 158–64.
36.
Goncerzewicz A, Misiewicz A. Wzbogacanie żywności kwasem folio-wym – naturalnym metabolitem przemysłowych szczepów drożdży saccharomyces cerevisie oraz bakterii fermentacji mlekowej. 2011: 20.
37.
Romero-Luna HE, Hernández-Sánchez H, Ribas-Aparicio RM, et al. Evaluation of the Probiotic Potential of Saccharomyces cerevisiae Strain (C41) Isolated from Tibicos by In Vitro Studies. Probiotics Antimicrob Proteins. 2019; 11: 794–800.
38.
Białecka-Florjańczyk E, Majewska E. Biotransformacje z udziałem drożdży Saccharomyces cerevisiae. Biotechnol 3. 2006: 113–33.
39.
Rodríguez-González EM, Muro-Medina CV, Arias A. Identificacion de levaduras aisladas de tepache casero y comercial. :1.
40.
Swangkeaw J, Vichitphan S, Butzke CE, et al. Characterization of β-glucosidases from Hanseniaspora sp. and Pichia anomala with poten-tially aroma-enhancing capabilities in juice and wine. World J Microbiol Biotechnol. 2011; 27: 423–30.
41.
Abd-El-Al ATH, Phaff HJ. Purification and properties of endo-β-glucanase in the yeast Hanseniaspora valbyensis. Can J Microbiol. 2011. doi: 10.1139/m69-123.
42.
Guatemala-Morales G, González P, Corona R, et al. The use of response surface methodology to evaluate the fermentation conditions in the production of tepache. Rev Mex Ing Quím. 2013; 12: 19–29.
43.
Dimidi E, Cox SR, Rossi M, et al. Fermented Foods: Definitions and Cha-racteristics, Impact on the Gut Microbiota and Effects on Gastrointe-stinal Health and Disease. Nutrients. 2019; 11. doi: 10.3390/nu11081806.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.