RESEARCH PAPER
Wstępny model oceny kosztów rzeczywistych ponoszonych przez podmiot leczniczy w związku z realizacją onkologicznego programu lekowego
1
Academy of Humanities and Economics, Łódź, Poland
Med Og Nauk Zdr. 2022;28(2):149-156
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
Modern drug therapies are made available on the basis of specific legal and economic solutions. In Poland, a model for financing modern therapies by the public payer in the form of a drug programme has been developed. The basic principles of such a strictly defined therapeutic procedure, apart from ensuring maximum safety and clinical effectiveness, is the need to define a budget that can be used for these usually expensive drugs. The aim of the study was to identify and analyse the actual costs related to the treatment process of a patient within the framework of an oncological drug programme, incurred by a healthcare entity.
Material and methods:
The oncological drug programme B.50 ‘Treatment of patients with ovarian cancer, fallopian tube cancer or peritoneal cancer’, implemented in an oncology centre in 2018–2021, was selected for the analysis. On average, 19 patients were treated annually.
Results:
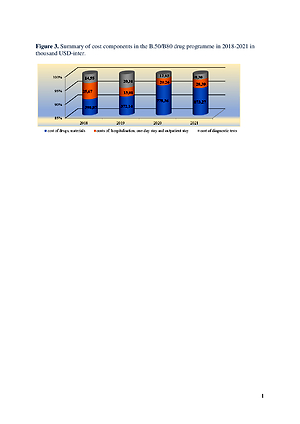
The lump-sum method of financing healthcare benefits under the drug programme, adopted by the public payer, does not cover the actual costs of treatment. Providing patients with all necessary medical services at every stage of the treatment process, which are not financed by the payer under the drug programme, creates a real risk of indebtedness to the healthcare entity.
Conclusions:
The chronic nature and therapeutic process of many diseases generate additional costs for the treatment of complications. Without the valuation of benefits adequate to the actual costs of treatment, a significant increase in the availability of innovative therapies to patients may become impossible.
Modern drug therapies are made available on the basis of specific legal and economic solutions. In Poland, a model for financing modern therapies by the public payer in the form of a drug programme has been developed. The basic principles of such a strictly defined therapeutic procedure, apart from ensuring maximum safety and clinical effectiveness, is the need to define a budget that can be used for these usually expensive drugs. The aim of the study was to identify and analyse the actual costs related to the treatment process of a patient within the framework of an oncological drug programme, incurred by a healthcare entity.
Material and methods:
The oncological drug programme B.50 ‘Treatment of patients with ovarian cancer, fallopian tube cancer or peritoneal cancer’, implemented in an oncology centre in 2018–2021, was selected for the analysis. On average, 19 patients were treated annually.
Results:
The lump-sum method of financing healthcare benefits under the drug programme, adopted by the public payer, does not cover the actual costs of treatment. Providing patients with all necessary medical services at every stage of the treatment process, which are not financed by the payer under the drug programme, creates a real risk of indebtedness to the healthcare entity.
Conclusions:
The chronic nature and therapeutic process of many diseases generate additional costs for the treatment of complications. Without the valuation of benefits adequate to the actual costs of treatment, a significant increase in the availability of innovative therapies to patients may become impossible.
REFERENCES (19)
1.
Wytyczne oceny technologii medycznych AOTMiT. https://www.aotm.gov.pl/media/... (access: 2022.02.27).
2.
Raport. Mechanizmy wczesnego dostępu do leków innowacyjnych na świecie ze szczególnym uwzględnieniem terapii onkologicznych. Warszawa: Instytut Zarządzania w Ochronie Zdrowia, Uczelnia Łazarskiego; 2016. https://izwoz.lazarski.pl/file... (access: 2022.03.01).
3.
Announcement of the Minister of Health of 21 February 2022 on the list of reimbursable medicines, foodstuffs for particular nutritional uses and medical devices as of 1 March 2022. https://www.gov.pl/web/zdrowie... (access: 2022.03.01).
5.
The Act of 12 May 2011 on the Reimbursement of Medicines, Foodstuffs for Particular Nutritional Uses and Medical Devices (Journal of Laws (Dziennik Ustaw) 2011, No. 122, item 696 as amended). https://isap.sejm. gov.pl/isap.nsf/download.xsp/WDU20111220696/U/D20110696Lj.pdf.
6.
Polityka Lekowa Państwa 2018–2022. https://www.gov.pl/web/ zdrowie/rada-ministrow-przyjela-dokument-polityka-lekowapanstwa-20182022 (access: 2022.02.17).
7.
Announcement of the Minister of Health of 21 April 2021 on the list of reimbursable medicines, foodstuffs for particular nutritional uses and medical devices as of 1 May 2021 https://www.gov.pl/web/ zdrowie/obwieszczenie-ministra-zdrowia z-dnia-21-kwietnia-2021- r-w-sprawie-wykazu-refundowanych-lekow-srodkow-spozywczychspecjalnego- przeznaczenia zywieniowego-oraz-wyrobow-medycznychna- 1-maja-2021-r (access: 2022.03.01).
8.
Order No. 20/2021/DSOZ of the President of the National Health Fund of 27 January 2021 amending the order on detailed terms and conditions of contracts in the system of basic hospital healthcare benefits https://www.nfz.gov.pl/zarzadz... zarzadzenie-nr-1622020dgl,7246.html.
9.
Purchasing power parities (PPP) https://data.oecd.org/conversi... purchasing-power-parities-ppp.htm.
10.
Patient-day – unit of measure used for reporting healthcare benefits by a medical institution in order to settle them with the payer – the National Health Fund (NFZ). It refers to the patient›s stay in an inpatient or day ward. The day of admitting the patient to treatment at the ward and the day of its completion are reported for settlement as one patient-day.
11.
Orlewska E. Reguły decyzyjne w ocenie ekonomicznej programów zdrowotnych. Farmakoekonomika 2004, No. 1.
12.
Stajszczyk M, Obarska I. Raport. Dostępność terapii i świadczeń w programach lekowych w chorobach autoimmunologicznych. Wpływ wprowadzenia ryczałtowego modelu opieki ambulatoryjnej na budżet płatnika publicznego. Warszawa: HealthCare System Navigator; 2021.
13.
Rogowski W, Zyśk R, Krzakowski M. Programy onkologiczne w onkologii. Jak skutecznie wykorzystać ich możliwości?. Onkol Prakt Klin Edu. 2018; 4(5): 321–333.
14.
Order No. 162/2020/DGL of the President of the National Health Fund of 16 October 2020 on lying down the terms and conditions for concluding and implementing contracts regarding hospital treatment in respect of drug programs https://www.nfz.gov.pl/zarzadz... zarzadzenia-prezesa-nfz/zarzadzenie-nr-1622020dgl,7246.html.
15.
The Act of 27 August 2004 on Healthcare Benefits Financed from Public Funds (Journal of Laws 2004 No. 210, item 2135 as amended). https://isap.sejm.gov.pl/isap.... D20042135Lj.pdf.
16.
Stajszczyk M. Rozliczanie kosztów realizacji programów lekowych – ważna zmiana na czas epidemii COVID-19. https://www.rynekzdrowia. pl/Finanse-i-zarzadzanie/Rozliczanie-kosztow-realizacji-programowlekowych- wazna-zmiana-na-czas-epidemii-COVID-19,212189,1.html (access: 2022.03.07).
17.
The Act of 14 August of 2020 on Amending Certain Acts to Ensure the Functioning of Health Care in Connection with the COVID-19 Epidemic and after its Cessation (Journal of Laws 2020, item 1493). https://isap.sejm.gov.pl/isap.... D20201493.pdf.
18.
Czech M, Gierczyński J, Jakubiak K, et al. Raport. Rozwój terapii lekowych w leczeniu chorych na nowotwory. Nowości, Innowacje, Przełomy. Modern Healthcare Institute, 2020.
19.
Rozliczanie diagnostyki i leczenia nowotworów w praktyce – poradnik dla placówek medycznych. Kielce: Polskie Towarzystwo Koderów Medycznych; 2020. https://ptkm.org.pl/media/atta... pt km_poradnik _roz l icz ani e_diagnos t yk i_i_lecz enia _ nowotworow_w_praktyce.pdf (access: 2022.03.12).
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.